Abstract
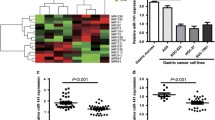
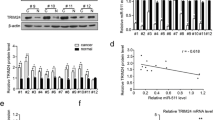
Dysregulation of microRNAs (miRNAs or miRs) is implicated in the development of gastric cancer (GC), which is possibly related to their roles in targeting tumor-suppressive or tumor-promoting genes. Herein, the current study was intended to ascertain the function of miR-488 and its modulatory mechanism in GC. Initially, human GC cells were assayed for their in vitro malignancy after miRNA gain- or loss-of-function and RNA interference or overexpression. Also, tumorigenesis and liver metastasis were evaluated in nude mouse models. Results demonstrated that miR-488 elevation suppressed GC (MKN-45 and OCUM-1) cell proliferation, migration, and invasiveness in vitro and reduced their tumorigenesis and liver metastasis in vivo. The luciferase assay identified that miR-488 bound to HULC and inhibited its expression. Furthermore, HULC could enhance EZH2-H3K27me3 enrichment at the p53 promoter region and epigenetically repress the p53 expression based on the data from RIP- and ChIP-qPCR assay. Additionally, HULC was validated to enhance GC growth and metastasis in vitro and in vivo. Overall, HULC re-expression elicited by miR-488 inhibition can enhance EZH2-H3K27me3 enrichment in the p53 promoter and repress the p53 expression, thus promoting the growth and metastasis of GC.
Graphical abstract







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Bardou P, Mariette J, Escudie F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293. https://doi.org/10.1186/1471-2105-15-293.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Cai Y, Li Y, Sun B, Wang H, Zhang W, Zhao Y, et al. LncRNA PTCSC3 and HULC negatively affect each other to regulate cancer cell invasion and migration in gastric cancer. Cancer Manag Res. 2020;12:8535–43. https://doi.org/10.2147/CMAR.S254944.
Campbell K. Contribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasis. Curr Opin Cell Biol. 2018;55:30–5. https://doi.org/10.1016/j.ceb.2018.06.008.
Chen Q, Zheng PS, Yang WT. EZH2-mediated repression of GSK-3beta and TP53 promotes Wnt/beta-catenin signaling-dependent cell expansion in cervical carcinoma. Oncotarget. 2016;7:36115–29. https://doi.org/10.18632/oncotarget.8741.
Chen S, Wu DD, Sang XB, Wang LL, Zong ZH, Sun KX, et al. The HULC functions as an oncogene by targeting ATG7 and ITGB1 in epithelial ovarian carcinoma. Cell Death Dis. 2017;8:e3118. https://doi.org/10.1038/cddis.2017.486.
Chen WM, Chen WD, Jiang XM, Jia XF, Wang HM, Zhang QJ, et al. HOX transcript antisense intergenic RNA represses E-cadherin expression by binding to EZH2 in gastric cancer. World J Gastroenterol. 2017;23:6100–10. https://doi.org/10.3748/wjg.v23.i33.6100.
Chen D, Zhou H, Liu G, Zhao Y, Cao G, Liu Q. SPOCK1 promotes the invasion and metastasis of gastric cancer through Slug-induced epithelial-mesenchymal transition. J Cell Mol Med. 2018;22:797–807. https://doi.org/10.1111/jcmm.13357.
Chen TH, Chiu CT, Lee C, Chu YY, Cheng HT, Hsu JT, et al. Circulating microRNA-22-3p predicts the malignant progression of precancerous gastric lesions from intestinal metaplasia to early adenocarcinoma. Dig Dis Sci. 2018;63:2301–8. https://doi.org/10.1007/s10620-018-5106-4.
Chen RY, Ju Q, Feng LM, Yuan Q, Zhang L. The carcinogenic complex lncRNA FOXP4-AS1/EZH2/LSD1 accelerates proliferation, migration and invasion of gastric cancer. Eur Rev Med Pharmacol Sci. 2019;23:8371–6. https://doi.org/10.26355/eurrev_201910_19148.
Cheng L, Wang P, Tian R, Wang S, Guo Q, Luo M, et al. LncRNA2Target v2.0: a comprehensive database for target genes of lncRNAs in human and mouse. Nucleic Acids Res. 2019;47:D140–4. https://doi.org/10.1093/nar/gky1051.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104. https://doi.org/10.1186/s13045-020-00937-8.
Feng A, Yuan X, Li X. MicroRNA-345 inhibits metastasis and epithelial-mesenchymal transition of gastric cancer by targeting FOXQ1. Oncol Rep. 2017;38:2752–60. https://doi.org/10.3892/or.2017.6001.
First EZH2 inhibitor approved-for rare sarcoma (2020) Cancer Discov 10 333 4. https://doi.org/10.1158/2159-8290.CD-NB2020-006
Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail. 2012;14:147–54. https://doi.org/10.1093/eurjhf/hfr155.
Gounder M, Schoffski P, Jones RL, Agulnik M, Cote GM, Villalobos VM, et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol. 2020;21:1423–32. https://doi.org/10.1016/S1470-2045(20)30451-4.
Gu Y, Chen T, Li G, Yu X, Lu Y, Wang H, et al. LncRNAs: emerging biomarkers in gastric cancer. Future Oncol. 2015;11:2427–41. https://doi.org/10.2217/fon.15.175.
Guan D, Li C, Li Y, Li Y, Wang G, Gao F, et al. The DpdtbA induced EMT inhibition in gastric cancer cell lines was through ferritinophagy-mediated activation of p53 and PHD2/hif-1alpha pathway. J Inorg Biochem. 2021;218:111413. https://doi.org/10.1016/j.jinorgbio.2021.111413.
Guo JY, Wang XQ, Sun LF. MicroRNA-488 inhibits ovarian cancer cell metastasis through regulating CCNG1 and p53 expression. Eur Rev Med Pharmacol Sci. 2020;24:2902–10. https://doi.org/10.26355/eurrev_202003_20654.
Han TS, Voon DC, Oshima H, Nakayama M, Echizen K, Sakai E, et al. Interleukin 1 up-regulates MicroRNA 135b to promote inflammation-associated gastric carcinogenesis in mice. Gastroenterology. 2019;156(1140–55):e4. https://doi.org/10.1053/j.gastro.2018.11.059.
Hu D, Shen D, Zhang M, Jiang N, Sun F, Yuan S, et al. MiR-488 suppresses cell proliferation and invasion by targeting ADAM9 and HULC in hepatocellular carcinoma. Am J Cancer Res. 2017;7:2070–80.
Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19:649–59. https://doi.org/10.1016/S1470-2045(18)30145-1.
Jiang X, Liu W. Long noncoding RNA highly upregulated in liver cancer activates p53–p21 pathway and promotes nasopharyngeal carcinoma cell growth. DNA Cell Biol. 2017;36:596–602. https://doi.org/10.1089/dna.2017.3686.
Jin C, Shi W, Wang F, Shen X, Qi J, Cong H, et al. Long non-coding RNA HULC as a novel serum biomarker for diagnosis and prognosis prediction of gastric cancer. Oncotarget. 2016;7:51763–72. https://doi.org/10.18632/oncotarget.10107.
Lee SY, Ju MK, Jeon HM, Lee YJ, Kim CH, Park HG, et al. Oncogenic metabolism acts as a prerequisite step for induction of cancer metastasis and cancer stem cell phenotype. Oxid Med Cell Longev. 2018;2018:1027453. https://doi.org/10.1155/2018/1027453.
Lin Y, Liu T, Cui T, Wang Z, Zhang Y, Tan P, et al. RNAInter in 2020: RNA interactome repository with increased coverage and annotation. Nucleic Acids Res. 2020;48:D189–97. https://doi.org/10.1093/nar/gkz804.
Liu J, Ben Q, Lu E, He X, Yang X, Ma J, et al. Long noncoding RNA PANDAR blocks CDKN1A gene transcription by competitive interaction with p53 protein in gastric cancer. Cell Death Dis. 2018;9:168. https://doi.org/10.1038/s41419-017-0246-6.
Mao X, Ji T, Liu A, Weng Y. ELK4-mediated lncRNA SNHG22 promotes gastric cancer progression through interacting with EZH2 and regulating miR-200c-3p/Notch1 axis. Cell Death Dis. 2021;12:957. https://doi.org/10.1038/s41419-021-04228-z.
Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9. https://doi.org/10.1038/nature09784.
Morenikeji OB, Bernard K, Strutton E, Wallace M, Thomas BN. Evolutionarily conserved long non-coding RNA regulates gene expression in cytokine storm during COVID-19. Front Bioeng Biotechnol. 2020;8:582953. https://doi.org/10.3389/fbioe.2020.582953.
Morschhauser F, Tilly H, Chaidos A, McKay P, Phillips T, Assouline S, et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020;21:1433–42. https://doi.org/10.1016/S1470-2045(20)30441-1.
Ouyang L, Yan B, Liu Y, Mao C, Wang M, Liu N, et al. The deubiquitylase UCHL3 maintains cancer stem-like properties by stabilizing the aryl hydrocarbon receptor. Signal Transduct Target Ther. 2020;5:78. https://doi.org/10.1038/s41392-020-0181-3.
Pan Y, Wu A, Xu F, Chen C, Jiang L, Jin R. Lentivirus-mediated overexpression of miR-124 suppresses growth and invasion by targeting JAG1 and EZH2 in gastric cancer. Oncol Lett. 2018;15:7450–8. https://doi.org/10.3892/ol.2018.8194.
Picao-Osorio J, Johnston J, Landgraf M, Berni J, Alonso CR. MicroRNA-encoded behavior in Drosophila. Science. 2015;350:815–20. https://doi.org/10.1126/science.aad0217.
Saidj D, Cros MP, Hernandez-Vargas H, Guarino F, Sylla BS, Tommasino M, et al. Oncoprotein E7 from beta human papillomavirus 38 induces formation of an inhibitory complex for a subset of p53-regulated promoters. J Virol. 2013;87:12139–50. https://doi.org/10.1128/JVI.01047-13.
Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437–48. https://doi.org/10.1016/S1470-2045(18)30739-3.
Sikand K, Slaibi JE, Singh R, Slane SD, Shukla GC. miR 488* inhibits androgen receptor expression in prostate carcinoma cells. Int J Cancer. 2011;129:810–9. https://doi.org/10.1002/ijc.25753.
Song Y, Wang R, Li LW, Liu X, Wang YF, Wang QX, et al. Long non-coding RNA HOTAIR mediates the switching of histone H3 lysine 27 acetylation to methylation to promote epithelial-to-mesenchymal transition in gastric cancer. Int J Oncol. 2019;54:77–86. https://doi.org/10.3892/ijo.2018.4625.
Sun H, Wang L, Zhao Q, Dai J. Diagnostic and prognostic value of serum miRNA-1290 in human esophageal squamous cell carcinoma. Cancer Biomark. 2019;25:381–7. https://doi.org/10.3233/CBM-190007.
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–52. https://doi.org/10.1093/nar/gku1003.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–13. https://doi.org/10.1093/nar/gky1131.
Tan D, Tan S, Zhang J, Tang P, Huang J, Zhou W, et al. Histone trimethylation of the p53 gene by expression of a constitutively active prolactin receptor in prostate cancer cells. Chin J Physiol. 2013;56:282–90. https://doi.org/10.4077/CJP.2013.BAB139.
Tanabe S, Aoyagi K, Yokozaki H, Sasaki H. Gene expression signatures for identifying diffuse-type gastric cancer associated with epithelial-mesenchymal transition. Int J Oncol. 2014;44:1955–70. https://doi.org/10.3892/ijo.2014.2387.
Tang Q, Su Z, Gu W, Rustgi AK. Mutant p53 on the path to metastasis. Trends Cancer. 2020;6:62–73. https://doi.org/10.1016/j.trecan.2019.11.004.
Tong HX, Zhou YH, Hou YY, Zhang Y, Huang Y, Xie B, et al. Expression profile of microRNAs in gastrointestinal stromal tumors revealed by high throughput quantitative RT-PCR microarray. World J Gastroenterol. 2015;21:5843–55. https://doi.org/10.3748/wjg.v21.i19.5843.
Wang P, Li Z, Liu H, Zhou D, Fu A, Zhang E. MicroRNA-126 increases chemosensitivity in drug-resistant gastric cancer cells by targeting EZH2. Biochem Biophys Res Commun. 2016;479:91–6. https://doi.org/10.1016/j.bbrc.2016.09.040.
Wang X, Jin Y, Zhang H, Huang X, Zhang Y, Zhu J. MicroRNA-599 inhibits metastasis and epithelial-mesenchymal transition via targeting EIF5A2 in gastric cancer. Biomed Pharmacother. 2018;97:473–80. https://doi.org/10.1016/j.biopha.2017.10.069.
Wang Y, Xiao H, Wang C, Wu H, He H, Yao C, et al. M-phase phosphoprotein 8 promotes gastric cancer growth and metastasis via p53/Bcl-2 and EMT-related signaling pathways. J Cell Biochem. 2020;121:2330–42. https://doi.org/10.1002/jcb.29456.
Wei GH, Wang X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:3850–6.
Wu F, Li J, Guo N, Wang XH, Liao YQ. MiRNA-27a promotes the proliferation and invasion of human gastric cancer MGC803 cells by targeting SFRP1 via Wnt/beta-catenin signaling pathway. Am J Cancer Res. 2017;7:405–16.
Wu S, Du R, Gao C, Kang J, Wen J, Sun T. The role of XBP1s in the metastasis and prognosis of hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;500:530–7. https://doi.org/10.1016/j.bbrc.2018.04.033.
Xu Z, Feng J, Li Y, Guan D, Chen H, Zhai X, et al. The vicious cycle between ferritinophagy and ROS production triggered EMT inhibition of gastric cancer cells was through p53/AKT/mTor pathway. Chem Biol Interact. 2020;328:109196. https://doi.org/10.1016/j.cbi.2020.109196.
Yang XJ, Huang CQ, Peng CW, Hou JX, Liu JY. Long noncoding RNA HULC promotes colorectal carcinoma progression through epigenetically repressing NKD2 expression. Gene. 2016;592:172–8. https://doi.org/10.1016/j.gene.2016.08.002.
Yang Y, Li H, He Z, Xie D, Ni J, Lin X. MicroRNA-488-3p inhibits proliferation and induces apoptosis by targeting ZBTB2 in esophageal squamous cell carcinoma. J Cell Biochem. 2019;120:18702–13. https://doi.org/10.1002/jcb.29178.
Zhao Y, Lu G, Ke X, Lu X, Wang X, Li H, et al. miR-488 acts as a tumor suppressor gene in gastric cancer. Tumour Biol. 2016;37:8691–8. https://doi.org/10.1007/s13277-015-4645-y.
Zheng L, Chen Y, Ye L, Jiao W, Song H, Mei H, et al. miRNA-584-3p inhibits gastric cancer progression by repressing Yin Yang 1- facilitated MMP-14 expression. Sci Rep. 2017;7:8967. https://doi.org/10.1038/s41598-017-09271-5.
Zhou KR, Liu S, Sun WJ, Zheng LL, Zhou H, Yang JH, et al. ChIPBase v2.0: decoding transcriptional regulatory networks of non-coding RNAs and protein-coding genes from ChIP-seq data. Nucleic Acids Res. 2017;45:D43–50. https://doi.org/10.1093/nar/gkw965.
Funding
This work was supported by Natural Science Foundation of Shanghai (No. 16ZR1436800 & 17ZR1439300), the First Program Features War Trauma Supported by Second Affiliated Hospital of Naval Medical University (Changzheng Hospital) (No. 201711015), and the Science and Technology Commission of Shanghai Municipality (No. 21140901200).
Author information
Authors and Affiliations
Contributions
D. J. Y. and Z. X. Z. conceived and designed research. D. J. Y., M. Y. S., and Z. X. Z. performed experiments. Q. Y. and Y. Z. analyzed data. Z. Q. H. and J. P. X. interpreted results of experiments. Q. P. C. prepared figures. D. J. Y., M. Y. S., Q. Y., and Y. Z. drafted manuscript. Z. Q. H., J. P. X., Q. P. C., and Z. X. Z. edited and revised manuscript. D. J. Y., M. Y. S., Q. Y., Y. Z., Z. Q. H., J. P. X., Q. P. C., and Z. X. Z. approved final version of manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The current study was approved by the Clinical Ethics Committee of the Second Affiliated Hospital of Naval Medical University. Animal studies involved in this paper were conducted conforming to the approval from the Institutional Animal Care and Use Committee of Second Affiliated Hospital of Naval Medical University.
Consent to participate
All participants signed informed consent documentation.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, D., Shi, M., You, Q. et al. Tumor- and metastasis-promoting roles of miR-488 inhibition via HULC enhancement and EZH2-mediated p53 repression in gastric cancer. Cell Biol Toxicol 39, 1341–1358 (2023). https://doi.org/10.1007/s10565-022-09760-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-022-09760-y