Abstract
Testicular Leydig cells are major contributors of androgen synthesis and secretion, which play an important role in testis development, normal masculinization, maintenance of spermatogenesis, and general male fertility. The rate-limiting step in testosterone biosynthesis involves the transfer of cholesterol to the mitochondrial inner membrane by the steroidogenic acute regulatory (Star) protein, a critical factor in steroid hormone biosynthesis. Once inside the mitochondria, cholesterol is metabolized by the steroidogenic enzyme Cyp11a1 to pregnenolone, which is further converted to testosterone by the action of other steroidogenic enzymes. Interestingly, the Star protein level declines during Leydig cell aging, resulting in defective mitochondrial cholesterol transfer and lower testosterone production. It is possible to delay the age-related decline in testosterone production by increasing Star and/or Cyp11a1 gene expression using supplementation with flavonoids, a group of polyphenolic compounds widely distributed in fruits and vegetables. In this study, we examined whether the distribution of hydroxyl groups among flavones could influence their potency to stimulate steroidogenesis within Leydig cells. Low levels of apigenin, luteolin, chrysin, and baicalein (10 μM) stimulated cAMP-dependent Star, Cyp11a1, and Fdx1 promoters’ activation and may increase steroidogenesis within Leydig cells. Indeed, luteolin effectively increased cAMP-dependent accumulation of progesterone from MA-10 Leydig cells, possibly through activation of Star and Fdx1 transcription. Thus, dietary luteolin could be potentially effective to maintain steroid production within aging males.









Similar content being viewed by others
Abbreviations
- bp:
-
Base pair
- cAMP:
-
Cyclic adenosine monophosphate
- Cebp:
-
CCAAT/enhancer binding protein
- Cox2:
-
Cyclooxygenase-2
- Creb:
-
cAMP response element binding protein
- Cyp11a1:
-
Cholesterol side-chain cleavage enzyme
- ELISA:
-
Enzyme-linked immunosorbent assay
- Dax1:
-
Dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1
- Fdx1:
-
Ferredoxin 1
- FSK:
-
Forskolin
- LH:
-
Luteinizing hormone
- NFkB:
-
Nuclear factor kappa B
- PKA:
-
Protein kinase A
- Star:
-
Steroidogenic acute regulatory protein
References
Ascoli M. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology. 1981;108(1):88–95.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Cárdenas M, Marder M, Blank VC, Roguin LP. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg Med Chem. 2006;14(9):2966–71.
Chen L-J, Games DE, Jones J. Isolation and identification of four flavonoid constituents from the seeds of Oroxylum indicum by high-speed counter-current chromatography. J Chromatogr A. 2003;988(1):95–105.
Chen C-Y, Peng W-H, Tsai K-D, Hsu S-L. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81(23–24):1602–14.
Choi EM, Lee YS. Luteolin suppresses IL-1beta-induced cytokines and MMPs production via p38 MAPK, JNK, NF-kappaB and AP-1 activation in human synovial sarcoma cell line, SW982. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2010;48(10):2607–11.
Chun K-S, Surh Y-J. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68(6):1089–100.
Cohen PG. Aromatase, adiposity, aging and disease. The hypogonadal-metabolic-atherogenic-disease and aging connection. Med Hypotheses. 2001;56(6):702–8.
Culty M, Luo L, Yao Z-X, Chen H, Papadopoulos V, Zirkin BR. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl. 2002;23(3):439–47.
Daigle M, Roumaud P, Martin LJ. Expressions of Sox9, Sox5, and Sox13 transcription factors in mice testis during postnatal development. Mol Cell Biochem. 2015;407(1–2):209–21.
De Maddalena C, Vodo S, Petroni A, Aloisi AM. Impact of testosterone on body fat composition. J Cell Physiol. 2012;227(12):3744–8.
Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol. 2006;65(1):125–31.
Dhawan K, Kumar S, Sharma A. Beneficial effects of chrysin and benzoflavone on virility in 2-year-old male rats. J Med Food. 2002;5(1):43–8.
Ferrándiz ML, Alcaraz MJ. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions. 1991;32(3–4):283–8.
Figueiroa MS, César Vieira JSB, Leite DS, Filho RCOA, Ferreira F, Gouveia PS, et al. Green tea polyphenols inhibit testosterone production in rat Leydig cells. Asian J. Androl. 2009;11(3):362–70.
Ha SK, Moon E, Kim SY. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett. 2010;485(3):143–7.
Hu G-X, Zhao B-H, Chu Y-H, Zhou H-Y, Akingbemi BT, Zheng Z-Q, et al. Effects of genistein and equol on human and rat testicular 3beta-hydroxysteroid dehydrogenase and 17beta-hydroxysteroid dehydrogenase 3 activities. Asian J Androl. 2010;12(4):519–26.
Hwang YP, Oh KN, Yun HJ, Jeong HG. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J Dermatol Sci. 2011;61(1):23–31.
Jana K, Yin X, Schiffer RB, Chen J-J, Pandey AK, Stocco DM, et al. Chrysin, a natural flavonoid enhances steroidogenesis and steroidogenic acute regulatory protein gene expression in mouse Leydig cells. J Endocrinol. 2008;197(2):315–23.
Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: a site-directed mutagenesis study. Environ Health Perspect. 1998;106(2):85–92.
Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221–7
Kellis JT, Vickery LE. Inhibition of human estrogen synthetase (aromatase) by flavones. Science. 1984;225(4666):1032–4.
Kilgore MW, Stocco DM. Initial characterization of a subclone of the MA-10 mouse Leydig tumor cell line. Endocrinology. 1989;124(3):1210–6.
King SR, LaVoie HA. Gonadal transactivation of STARD1, CYP11A1 and HSD3B. Front Biosci Landmark Ed. 2012;17:824–46.
Leers-Sucheta S, Stocco DM, Azhar S. Down-regulation of steroidogenic acute regulatory (StAR) protein in rat Leydig cells: implications for regulation of testosterone production during aging. Mech Ageing Dev. 1999;107(2):197–203.
Li W, Pandey AK, Yin X, Chen J-J, Stocco DM, Grammas P, et al. Effects of apigenin on steroidogenesis and steroidogenic acute regulatory gene expression in mouse Leydig cells. J Nutr Biochem. 2011;22(3):212–8.
Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20(10):1945–52.
Lin D, Sugawara T, Strauss JF, Clark BJ, Stocco DM, Saenger P, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267(5205):1828–31.
Liu C, Wu J, Gu J, Xiong Z, Wang F, Wang J, et al. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2007;86(3):423–30.
Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, et al. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112(6):1500–12.
López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009;9(1):31–59.
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81(1 Suppl):230S–42S.
Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, et al. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol. Endocrinol. Baltim. Md. 2002;16(1):184–99.
Manna PR, Wang X-J, Stocco DM. Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids. 2003;68(14):1125–34.
Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod. 2009;15(6):321–33.
Mårin P. Testosterone and regional fat distribution. Obes Res. 1995;3(Suppl 4):609S–12S.
Mårin P, Arver S. Androgens and abdominal obesity. Baillières Clin Endocrinol Metab. 1998;12(3):441–51.
Martin LJ, Boucher N, Brousseau C, Tremblay JJ. The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through cooperation with Ca2+/calmodulin-dependent protein kinase I. Mol. Endocrinol. Baltim. Md. 2008;22(9):2021–37.
Martin LJ, Boucher N, El-Asmar B, Tremblay JJ. cAMP-induced expression of the orphan nuclear receptor Nur77 in MA-10 Leydig cells involves a CaMKI pathway. J Androl. 2009;30(2):134–45.
Mendoza-Villarroel RE, Robert NM, Martin LJ, Brousseau C, Tremblay JJ. The nuclear receptor NR2F2 activates star expression and steroidogenesis in mouse MA-10 and MLTC-1 Leydig cells. Biol Reprod. 2014;91(1):26.
Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K, et al. Luteolin as an anti-inflammatory and neuroprotective agent: a brief review. Brain Res Bull. 2015;119(Pt A):1–11.
Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, Sandström B, et al. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr. 1999;81(6):447–55.
Nishioka T, Kawabata J, Aoyama Y. Baicalein, an alpha-glucosidase inhibitor from Scutellaria baicalensis. J Nat Prod. 1998;61(11):1413–5.
Roumaud P, Martin LJ. Roles of leptin, adiponectin and resistin in the transcriptional regulation of steroidogenic genes contributing to decreased Leydig cells function in obesity. Horm Mol Biol Clin Investig. 2015;24(1):25–45.
Shin SI, Yasumura Y, Sato GH. Studies on interstitial cells in tissue culture. II. Steroid biosynthesis by a clonal line of rat testicular interstitial cells. Endocrinology. 1968;82(3):614–6.
Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221–44.
Swinnen JV, D’Souza B, Conti M, Ascoli M. Attenuation of cAMP-mediated responses in MA-10 Leydig tumor cells by genetic manipulation of a cAMP-phosphodiesterase. J Biol Chem. 1991;266(22):14383–9.
Tan RS, Pu SJ. Impact of obesity on hypogonadism in the andropause. Int J Androl. 2002;25(4):195–201.
Tremblay JJ, Viger RS. Transcription factor GATA-4 enhances Müllerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol Baltim Md. 1999;13(8):1388–401.
Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142(3):977–86.
Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38(10):1654–61.
Tuorkey MJ. Molecular targets of luteolin in cancer. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 2016;25(1):65–76.
Wang XJ. Natural flavonoids in StAR gene expression and testosterone biosynthesis in Leydig cell aging [Internet]. InTech. 2011. Available from: http://cdn.intechopen.com/pdfs/21807.pdf
Wang X, Liu Z, Eimerl S, Timberg R, Weiss AM, Orly J, et al. Effect of truncated forms of the steroidogenic acute regulatory protein on intramitochondrial cholesterol transfer. Endocrinology. 1998;139(9):3903–12.
Wang X, Shen C-L, Dyson MT, Eimerl S, Orly J, Hutson JC, et al. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology. 2005;146(10):4202–8.
Xiong Z, Jiang B, Wu P-F, Tian J, Shi L-L, Gu J, et al. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharm Bull. 2011;34(2):253–9.
Xu X, De Pergola G, Björntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126(2):1229–34.
Yao Y, Sang W, Zhou M, Ren G. Phenolic composition and antioxidant activities of 11 celery cultivars. J Food Sci. 2010;75(1):C9–13.
Yi Lau GT, Leung LK. The dietary flavonoid apigenin blocks phorbol 12-myristate 13-acetate-induced COX-2 transcriptional activity in breast cell lines. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010;48(10):3022–7.
Acknowledgements
The current work was funded by the New Brunswick Innovation Foundation (NBIF) (#IAR2015 to L.J.M.; #RIF2015-014 to M.T.) and the Natural Sciences and Engineering Research Council (NSERC) of Canada (#386557 to L.J.M.; #04560 to M.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
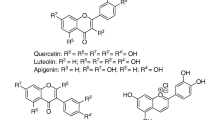
. Chemical structures of the flavones apigenin, luteolin, chrysin and baicalein. (PDF 33 kb)
Rights and permissions
About this article
Cite this article
Cormier, M., Ghouili, F., Roumaud, P. et al. Influences of flavones on cell viability and cAMP-dependent steroidogenic gene regulation in MA-10 Leydig cells. Cell Biol Toxicol 34, 23–38 (2018). https://doi.org/10.1007/s10565-017-9395-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-017-9395-8