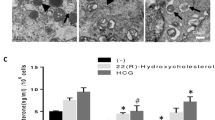
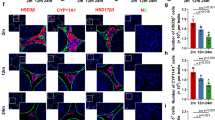
Abstract
Background
Mitochondrial dysfunction may contribute to decreased testosterone synthesis in aged Leydig cells. Resveratrol (RSV) as an antioxidant has been shown to exhibit multiple positive effects on mitochondrion, where steroidogenesis takes place. Whether RSV can improve steroidogenesis in aged testis is still unknown. This study investigates the effect of RSV on testosterone production during aging and corresponding changes in mitochondrial biogenesis and autophagy activity, which are closely associated with steroidogenesis. Whether ATG7, an important autophagy-related protein, functions in RSV-treated aged Leydig cells will also be explored.
Methods and results
Two-month-old male C57BL/6 mice were fed for 16 months by customized regular diet with or without RSV as diet supplement. Leydig cell line TM3 cells were treated with D-galactose to induce senescence, followed with or without RSV treatment. Results found that RSV supplement increased testosterone production in both aged mice and D-galactose-induced senescent Leydig cells. Western blot results revealed that RSV treatment elevated levels of steroidogenic rate-limiting enzymes StAR and 3β-HSD, as well as autophagy-related proteins LC3II, Beclin1, ATG5 and ATG7 and mitochondrial function-related proteins mtTFA and COXIV. However, after Atg7 was knocked down in senescent Leydig cells, even though RSV was added, levels of these proteins declined significantly, accompanied by decreased levels of mitochondrial transcript factors PGC-1α, mtTFA and NRF-1 and more fragmented mitochondria, demonstrating that Atg7 knockdown wrecked the protective effects of RSV on steroidogenesis in senescent Leydig cells.
Conclusion
ATG7-dependent autophagy plays a key role in RSV-brought testosterone production increase through regulating mitochondrial biogenesis in senescent Leydig cells.





Similar content being viewed by others
Data availability
The data and materials are available from the corresponding authors upon request.
References
Ketchem JM, Bowman EJ, Isales CM (2023) Male sex hormones, aging, and inflammation. Biogerontology 24:1–25. https://doi.org/10.1007/s10522-022-10002-1
Li WR, Chen L, Chang ZJ et al (2011) Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J Androl 13:881–888. https://doi.org/10.1038/aja.2011.85
Ramisz G, Turek W, Chmurska-Gasowska M et al (2021) Senescence and adiponectin signaling - studies in canine testis. Ann Anat 234:151606. https://doi.org/10.1016/j.aanat.2020.151606
Palmeira CM, Teodoro JS, Amorim JA et al (2019) Mitohormesis and metabolic health: the interplay between ros, camp and sirtuins. Free Radic Biol Med 141:483–491. https://doi.org/10.1016/j.freeradbiomed.2019.07.017
Miller WL (2013) Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol 379:62–73. https://doi.org/10.1016/j.mce.2013.04.014
Allen JA, Shankara T, Janus P et al (2006) Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology 147:3924–3935. https://doi.org/10.1210/en.2005-1204
Rubinsztein DC, Marino G, Kroemer G (2011) Autophagy and aging. Cell 146:682–695. https://doi.org/10.1016/j.cell.2011.07.030
Gao F, Li G, Liu C et al (2018) Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J Cell Biol 217:2103–2119. https://doi.org/10.1083/jcb.201710078
Herranz N, Gil J (2018) Mechanisms and functions of cellular senescence. J Clin Invest 128:1238–1246. https://doi.org/10.1172/JCI95148
Price NL, Gomes AP, Ling AJ et al (2012) Sirt1 is required for ampk activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab 15:675–690. https://doi.org/10.1016/j.cmet.2012.04.003
Yang X, Liu Q, Li Y et al (2020) The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the ampk-sirt1-pgc-1alpha signalling pathway. Adipocyte 9:484–494. https://doi.org/10.1080/21623945.2020.1807850
Palikaras K, Lionaki E, Tavernarakis N (2015) Coordination of mitophagy and mitochondrial biogenesis during ageing in c. Elegans Nat 521:525–528. https://doi.org/10.1038/nature14300
Schiavi A, Ventura N (2014) The interplay between mitochondria and autophagy and its role in the aging process. Exp Gerontol 56:147–153. https://doi.org/10.1016/j.exger.2014.02.015
Palikaras K, Daskalaki I, Markaki M et al (2017) Mitophagy and age-related pathologies: development of new therapeutics by targeting mitochondrial turnover. Pharmacol Ther 178:157–174. https://doi.org/10.1016/j.pharmthera.2017.04.005
Sabouny R, Shutt TE (2020) Reciprocal regulation of mitochondrial fission and fusion. Trends Biochem Sci 45:564–577. https://doi.org/10.1016/j.tibs.2020.03.009
Ding M, Feng N, Tang D et al (2018) Melatonin prevents drp1-mediated mitochondrial fission in diabetic hearts through sirt1-pgc1alpha pathway. J Pineal Res 65:e12491. https://doi.org/10.1111/jpi.12491
Yamada Y, Takano Y, Satrialdi et al (2020) Therapeutic strategies for regulating mitochondrial oxidative stress. Biomolecules. https://doi.org/10.3390/biom10010083
Hajam YA, Rani R, Ganie SY et al (2022) Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11:552. https://doi.org/10.3390/cells11030552
Lagouge M, Argmann C, Gerhart-Hines Z et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating sirt1 and pgc-1alpha. Cell 127:1109–1122. https://doi.org/10.1016/j.cell.2006.11.013
Madeo F, Carmona-Gutierrez D, Hofer SJ et al (2019) Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab 29:592–610. https://doi.org/10.1016/j.cmet.2019.01.018
Wang P, Lin M, Chen C et al (2022) Autophagy modulation in resveratrol protective effects on steroidogenesis in high-fat diet-fed mice and h2o2-challenged tm3 cells. Mol Biol Rep 49:2973–2983. https://doi.org/10.1007/s11033-022-07120-x
Wang HJ, Wang Q, Lv ZM et al (2015) Resveratrol appears to protect against oxidative stress and steroidogenesis collapse in mice fed high-calorie and high-cholesterol diet. Andrologia 47:59–65. https://doi.org/10.1111/and.12231
Beattie MC, Adekola L, Papadopoulos V et al (2015) Leydig cell aging and hypogonadism. Exp Gerontol 68:87–91. https://doi.org/10.1016/j.exger.2015.02.014
Tena-Sempere M, Rannikko A, Kero J et al (1997) Molecular mechanisms of reappearance of luteinizing hormone receptor expression and function in rat testis after selective Leydig cell destruction by ethylene dimethane sulfonate. Endocrinology 138:3340–3348. https://doi.org/10.1210/endo.138.8.5325
Wang P, Zhang S, Lin S et al (2022) Melatonin ameliorates diabetic hyperglycaemia-induced impairment of leydig cell steroidogenic function through activation of sirt1 pathway. Reprod Biol Endocrinol 20:117. https://doi.org/10.1186/s12958-022-00991-6
Wang LF, Cao Q, Wen K et al (2019) Cd38 deficiency alleviates d-galactose-induced myocardial cell senescence through nad+/sirt1 signaling pathway. Front Physiol 10:1125. https://doi.org/10.3389/fphys.2019.01125
Texada MJ, Malita A, Christensen CF et al (2019) Autophagy-mediated cholesterol trafficking controls steroid production. Dev Cell 48:659–671. https://doi.org/10.1016/j.devcel.2019.01.007
Zirkin BR, Chen H (2000) Regulation of Leydig cell steroidogenic function during aging. Biol Reprod 63:977–981. https://doi.org/10.1095/biolreprod63.4.977
Papadopoulos V, Zirkin BR (2021) Leydig cell aging: molecular mechanisms and treatments. Vitam Horm 115:585–609. https://doi.org/10.1016/bs.vh.2020.12.023
Novakovic R, Rajkovic J, Gostimirovic M et al (2022) Resveratrol and reproductive health. Life (Basel). https://doi.org/10.3390/life12020294
Neaves WB, Johnson L, Porter JC et al (1984) Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab 59:756–763. https://doi.org/10.1210/jcem-59-4-756
Kaufman JM, Lapauw B, Mahmoud A et al (2019) Aging and the male reproductive system. Endocr Rev 40:906–972. https://doi.org/10.1210/er.2018-00178
Pawlicki P, Koziorowska A, Koziorowski M et al (2023) Senescence and autophagy relation with the expressional status of non-canonical estrogen receptors in testes and adrenals of roe deer (Capreoluscapreolus) during the pre-rut period. Theriogenology 198:141–152. https://doi.org/10.1016/j.theriogenology.2022.12.023
Lustofin K, Niedbala P, Pawlicki P et al (2021) Senescent cells in rabbit, nutria and chinchilla testes-results from histochemical and immunohistochemical studies. Anim Reprod Sci 226:106701. https://doi.org/10.1016/j.anireprosci.2021.106701
Davinelli S, De Stefani D, De Vivo I et al (2020) Polyphenols as caloric restriction mimetics regulating mitochondrial biogenesis and mitophagy. Trends Endocrinol Metab 31:536–550. https://doi.org/10.1016/j.tem.2020.02.011
Chen M, Yan R, Luo J et al (2023) The role of pgc-1alpha-mediated mitochondrial biogenesis in neurons. Neurochem Res. https://doi.org/10.1007/s11064-023-03934-8
Qi XM, Qiao YB, Zhang YL et al (2023) Pgc-1alpha/nrf1-dependent cardiac mitochondrial biogenesis: a druggable pathway of calycosin against triptolide cardiotoxicity. Food Chem Toxicol 171:113513. https://doi.org/10.1016/j.fct.2022.113513
Cao W, Li J, Yang K et al (2021) An overview of autophagy: mechanism, regulation and research progress. Bull Cancer 108:304–322. https://doi.org/10.1016/j.bulcan.2020.11.004
Walczak-Jedrzejowska R, Slowikowska-Hilczer J, Marchlewsk K et al (2007) During seminiferous tubule maturation testosterone and synergistic action of fsh with estradiol support germ cell survival while estradiol alone has pro-apoptotic effect. Folia Histochem Cytobiol 45(Suppl 1):S59–S64
Wang Y, Chen F, Ye L et al (2017) Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction 154:R111–R122. https://doi.org/10.1530/REP-17-0064
Funding
This work was supported by supported by Anhui Provincial Natural Science Foundation of China (Grant No. 2108085MH259).
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All experiments were approved by Experimental Animal Ethics Committee of Anhui Medical University (NO. LLSC20200078) and followed the guidelines of the Administration of Affairs Concerning Animal Experimentation of China.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, F., Zhang, S., Zhu, X. et al. Autophagy-related 7 protein-dependent autophagy mediates resveratrol-caused upregulation of mitochondrial biogenesis and steroidogenesis in aged Leydig cell. Mol Biol Rep 51, 28 (2024). https://doi.org/10.1007/s11033-023-08935-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-08935-y