Abstract
Among numerous semiconductors, BiVO4 has a suitable bandgap width, excellent valence band energy level and high activity for visible light water oxidation. It is an important oxygen evolution photocatalyst, but its carrier migration rate is still lower than other semiconductor materials. Therefore, in this paper, transition metal cations such as Fe3+ were selectively exchanged with V5+ in BiVO4 in a high-temperature Ar atmosphere by vapor phase cation exchange method, cationic bonds with strong binding force were formed at the interface between metal elements and catalysts, effectively accelerating bulk to surface charge separation. The adsorption energy and surface oxidation kinetics of the intermediate in the process of water oxidation were enhanced, and the photoelectrochemical properties of the intermediate were effectively improved.
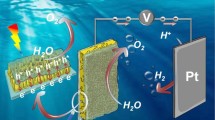
Graphical Abstract
The vapor phase cation exchange between Fe3+ and V5+ of BiVO4 photoanode makes the metal element and the catalyst form a strong cationic bond at the interface, which greatly reduces the charge transport resistance to a certain extent, accelerates the water reaction kinetics, which can not only significantly improve the stability, but also increase the photocurrent density and significantly reduce the starting potential.






Similar content being viewed by others
References
Kudo A, Ueda K, Kato H, Mikami I (1998) Catal Lett 53:229–230
Sayama K, Nomura A, Zou Z, Abe R, Abe Y, Arakawa H (2003) Chem Commun 23:2908–2909
Kim T-W, Choi K-S (2014) Science 343:990–994
Yang L, Fan D, Li Z, Cheng Y, Yang X, Zhang T (2022) Adv Sustainable Syst 6:2100477
Li X, Garlisi C, Guan Q, Anwer S, Al-Ali K, Palmisano G, Zheng L (2021) Mater Today 47:75–107
Fu J, Xu Q, Low J, Jiang C, Yu J (2019) Appl Catal B Environ 243:556–565
Su T, Shao Q, Qin Z, Guo Z, Wu Z (2018) ACS Catal 8:2253–2276
Zheng X, Kuang Q, Yan K, Qiu Y, Qiu J, Yang S (2013) ACS Appl Mater Inter 5:11249–11257
Abdi F-F, Han L, Smets A-H, Zeman M, Dam B, Krol R (2013) Nat Commun 4:2195
Li R, Zhang F, Wang D, Yang J, Li M, Zhu J, Zhou X, Han H, Li C (2013) Nat Commun 4:1432
Lightcap I-V, Kosel T-H, Kamat P-V (2010) Nano Lett 10:577–583
Lin Y, Zhang K, Chen W, Liu Y, Geng Z, Zeng J, Pan N, Yan L, Wang X, Hou J-G (2010) ACS Nano 4:3033–3038
Nair V, Perkins C-L, Lin Q, Law M (2016) Energ Environ Sci 9:1412–1429
Zhu J, Fan F, Chen R, An H, Feng Z, Li C (2015) Angew Chem Int Edit 54:9111–9114
Rajeshwar K, Tacconi NR, Chenthamarakshan C-R (2001) Chem Mater 13:2765–2782
Zhu S-S, Zhang Y, Zou Y, Guo S-Y, Liu H, Wang J-J, Braun A (2021) J Phys Chem C 125:15890–15898
Ding J-R, Kim K-S (2018) Chem Eng J 334:1650–1656
Chatchai P, Murakami Y, Kishioka S, Nosaka A-Y, Nosaka Y (2009) Electrochim Acta 54:1147–1152
Kim J-H, Yoon J-W, Kim T-H, Jo Y-M, Kim J-S, Jeong S-Y, Lee J-H (2021) Chem Eng J 425:131496
Nomellini C, Polo A, Mesa C-A, Pastor E, Marra G, Grigioni I, Dozzi M-V, Giménez S, Selli E (2023) ACS Appl Mater Inter 15:52436–52447
Zhang J, Huang Y, Lu X, Yang J, Tong Y (2021) ACS Sustain Chem Eng 9:8306–8314
Wang S, Cui D, Hao W, Du Y (2022) Energ Fuel 36:11394–11403
Meng L, Lv Z, Xu W, Tian W, Li L (2023) Adv Sci 10:2206729
Parmar K-P, Kang H-J, Bist A, Dua P, Jang J-S, Lee J-S (2012) Chemsuschem 5:1926–1934
He B, Li Z, Zhao D, Liu H, Zhong Y, Ning J, Zhang Z, Wang Y, Hu Y (2018) ACS Appl Nano Mater 1:2589–2599
Xia T, Chen M, Xiao L, Fan W, Mao B, Xu D, Guan P, Zhu J, Shi W (2018) J Taiwan Inst Chem E 93:582–589
Lee J-M, Baek J-H, Gill T-M, Shi X, Lee S, Cho I-S, Jung H-S, Zheng X-A (2019) J Mater Chem A 7:9019–9024
Shim S-G, Tan J, Lee H, Park J, Yun J, Park Y-S, Kim K, Lee J, Moon J (2022) Chem Eng J 430:133061
Zhang Y, Han W, Ding L, Fang F, Xie Z, Liu X, Chang K (2022) Catal Sci Technol 12:4040–4049
Zhang B, Zhang H, Wang Z, Zhang X, Qin X, Dai Y, Liu Y, Wang P, Li Y, Huang B (2017) Appl Catal B Environ 211:258–265
Polo A, Dozzi M-V, Grigioni I, Lhermitte C, Plainpan N, Moretti L, Cerullo G, Sivula K, Selli E (2022) Solar RRL 6:2200349
Nawaz A, Khan A, Ali N, Mao P, Gao X, Ali N, Bilal M, Khan H (2022) Chemosphere 289:133121
Wang S, He T, Chen P, Du A, Ostrikov K, Huang W, Wang L (2020) Adv Mater 32:2070198
Acknowledgements
This work was supported by the National Natural Science Foundation of Jiangsu Province (Grant No. BK20210308), the Basic Science Center Program for Ordered Energy Conversion of the National Natural Science Foundation of China (Grant No. 51888103) and the Fundamental Research Funds for the Central Universities (No. NE2019103).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Ding, L., Zhang, Y. et al. Insight of water oxidation kinetics of BiVO4 photoanode by vapor phase cation exchange method. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04673-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04673-3