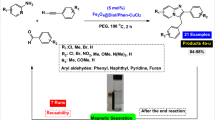
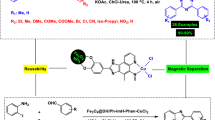
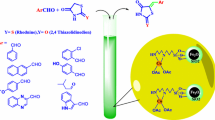
Abstract
Diaryl sulfides are known as valuable intermediates in the production of various useful compounds, including antibacterial, antifungal, anti-ulcer and anti-hypertensive agents. In this paper, we developed a new and ecofriendly nanomagnetic catalyst for the synthesis of diaryl sulfides containing imidazo[1,2-a]pyridine, benzoazole, pyrimidine and oxadiazole scaffolds through C–H bond sulfenylation of imidazopyridines. Fe3O4@AMBA–CuI nanocatalyst was successfully constructed through the immobilization of CuI on the surface of magnetic Fe3O4 nanoparticles modified with 3-amino-4-mercaptobenzoic acid (AMBA). FT-IR spectroscopy, SEM, EDX, TEM, XRD, VSM, EDX elemental mapping and ICP-OES techniques were applied to characterize the structure of the as-fabricated Fe3O4@AMBA–CuI nanocatalyst. Recycling of the Fe3O4@AMBA–CuI nanocatalyst was performed up to eight times without significant loss in activity; VSM and ICP-OES techniques confirmed that the stability and high magnetic nature of recovered catalyst.
Graphical Abstract















Similar content being viewed by others
References
Fanou GD, Traore M, Yao BK et al (2021) Photocatalytic activity of TiO2–P25@n-TiO2@HAP composite films for air depollution. Environ Sci Pollut Res 28:21326–21333. https://doi.org/10.1007/s11356-020-11924-4
Li W, Zheng Y, Qu E et al (2021) β-Keto amides: a jack-of-all-trades building block in organic chemistry. Eur J Org Chem 2021:5151–5192. https://doi.org/10.1002/ejoc.202100692
Nikseresht A, Bagherinia R, Mohammadi M, Mehravar R (2023) Phosphomolybdic acid hydrate encapsulated in MIL-53 (Fe): a novel heterogeneous heteropoly acid catalyst for ultrasound-assisted regioselective nitration of phenols. RSC Adv 13:674–687. https://doi.org/10.1039/D2RA07077D
Ibrahim M, Labaki M, Giraudon J-M, Lamonier J-F (2020) Hydroxyapatite, a multifunctional material for air, water and soil pollution control: a review. J Hazard Mater 383:121139. https://doi.org/10.1016/j.jhazmat.2019.121139
Mondal S, Samanta S, Hajra A (2018) Regioselective C-7 nitration of 8-aminoquinoline amides using tert-butyl nitrite. Adv Synth Catal 360:1026–1031. https://doi.org/10.1002/adsc.201701555
Ghobakhloo F, Mohammadi M, Mi G, Azarifar D (2023) Post-synthetic generation of amino-acid-functionalized UiO-66-NH2 metal-organic framework nanostructures as an amphoteric catalyst for organic reactions. ACS Appl Nano Mater. https://doi.org/10.1021/acsanm.3c05230
Mohammadi M, Khodamorady M, Tahmasbi B et al (2021) Boehmite nanoparticles as versatile support for organic–inorganic hybrid materials: synthesis, functionalization, and applications in eco-friendly catalysis. J Ind Eng Chem 97:1–78. https://doi.org/10.1016/j.jiec.2021.02.001
Ghobakhloo F, Azarifar D, Mohammadi M et al (2022) Copper(II) Schiff-base complex modified UiO-66-NH2(Zr) metal-organic framework catalysts for Knoevenagel condensation-Michael addition-cyclization reactions. Inorg Chem 61:4825–4841. https://doi.org/10.1021/acs.inorgchem.1c03284
Ghorbani-Choghamarani A, Taherinia Z, Mohammadi M (2021) Facile synthesis of Fe3O4@GlcA@Ni-MOF composites as environmentally green catalyst in organic reactions. Environ Technol Innov 24:102050. https://doi.org/10.1016/j.eti.2021.102050
Ghorbani-Choghamarani A, Mohammadi M, Tamoradi T, Ghadermazi M (2019) Covalent immobilization of Co complex on the surface of SBA-15: green, novel and efficient catalyst for the oxidation of sulfides and synthesis of polyhydroquinoline derivatives in green condition. Polyhedron 158:25–35. https://doi.org/10.1016/j.poly.2018.10.054
Li J, Wang Z, Ma Y et al (2023) Synthesis of mesoporous silica-supported NiCo bimetallic nanocatalysts and their enhanced catalytic hydrogenation performance. ACS Omega 8:12339–12347. https://doi.org/10.1021/acsomega.3c00076
Thomas N, Dionysiou DD, Pillai SC (2021) Heterogeneous Fenton catalysts: a review of recent advances. J Hazard Mater 404:124082. https://doi.org/10.1016/j.jhazmat.2020.124082
Chaudhari MB, Gnanaprakasam B (2019) Recent advances in the metal-catalyzed activation of amide bonds. Chem Asian J 14:76–93. https://doi.org/10.1002/asia.201801317
Li Z, Wang L, Qin L et al (2021) Recent advances in the application of water-stable metal-organic frameworks: adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 285:131432. https://doi.org/10.1016/j.chemosphere.2021.131432
Rai P, Gupta D (2021) Magnetic nanoparticles as green catalysts in organic synthesis—a review. Synth Commun 51:3059–3083. https://doi.org/10.1080/00397911.2021.1968910
Dehbanipour Z, Mongashti A (2022) The efficient heterogeneous catalyst containing copper(II) bis-benzothiazole complex supported on functionalized magnetic nanoparticles used for epoxidation of alkenes with tert-BuOOH. J Mol Struct 1265:133364. https://doi.org/10.1016/j.molstruc.2022.133364
Hudson R, Feng Y, Varma RS, Moores A (2014) Bare magnetic nanoparticles: sustainable synthesis and applications in catalytic organic transformations. Green Chem 16:4493–4505. https://doi.org/10.1039/C4GC00418C
Huang Y, Zhang W (2013) Magnetic nanoparticle-supported organocatalysis. Green Process Synthesis 2:603–609. https://doi.org/10.1515/gps-2013-0076
Dalpozzo R (2015) Magnetic nanoparticle supports for asymmetric catalysts. Green Chem 17:3671–3686. https://doi.org/10.1039/C5GC00386E
Kazemi M, Mohammadi M (2020) Magnetically recoverable catalysts: catalysis in synthesis of polyhydroquinolines. Appl Organomet Chem 34:e5400. https://doi.org/10.1002/aoc.5400
Mohammadi M, Ghorbani-Choghamarani A (2022) Complexation of guanidino containing l-arginine with nickel on silica-modified Hercynite MNPs: a novel catalyst for the Hantzsch synthesis of polyhydroquinolines and 2,3-Dihydroquinazolin-4(1H)-ones. Res Chem Intermed 48:2641–2663. https://doi.org/10.1007/s11164-022-04706-9
Khashei Siuki H, Ghamari Kargar P, Bagherzade G (2022) New acetamidine Cu(II) Schiff base complex supported on magnetic nanoparticles pectin for the synthesis of triazoles using click chemistry. Sci Rep 12:3771. https://doi.org/10.1038/s41598-022-07674-7
Sharma RK, Dutta S, Sharma S et al (2016) Fe3O4 (iron oxide)-supported nanocatalysts: synthesis, characterization and applications in coupling reactions. Green Chem 18:3184–3209. https://doi.org/10.1039/C6GC00864J
Zhang Q, Yang X, Guan J (2019) Applications of magnetic nanomaterials in heterogeneous catalysis. ACS Appl Nano Mater 2:4681–4697. https://doi.org/10.1021/acsanm.9b00976
Kamel Ariffin MF, Idris A (2022) Fe2O3/chitosan coated superparamagnetic nanoparticles supporting lipase enzyme from Candida antarctica for microwave assisted biodiesel production. Renew Energy 185:1362–1375. https://doi.org/10.1016/j.renene.2021.11.077
Shylesh S, Schünemann V, Thiel WR (2010) Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Angew Chem Int Ed 49:3428–3459. https://doi.org/10.1002/anie.200905684
Norouzi M, Noormoradi N, Mohammadi M (2023) Nanomagnetic tetraaza (N4 donor) macrocyclic Schiff base complex of copper(II): synthesis, characterizations, and its catalytic application in click reaction. Nanoscale Adv. https://doi.org/10.1039/D3NA00580A
Kheilkordi Z, Mohammadi Ziarani G, Mohajer F et al (2022) Recent advances in the application of magnetic bio-polymers as catalysts in multicomponent reactions. RSC Adv 12:12672–12701. https://doi.org/10.1039/D2RA01294D
Cheng T, Zhang D, Li H, Liu G (2014) Magnetically recoverable nanoparticles as efficient catalysts for organic transformations in aqueous medium. Green Chem 16:3401–3427. https://doi.org/10.1039/C4GC00458B
Aqeel Ashraf M, Liu Z, Yang Y, Zhang D (2020) Magnetic nanoparticles supported copper catalysts: synthesis of heterocyclic scaffolds. Synth Commun 50:2885–2905. https://doi.org/10.1080/00397911.2020.1789167
Aqeel Ashraf M, Liu Z, Yang Y et al (2020) Magnetic nanomaterials catalyzed synthesis of tetrazoles. Synth Commun 50:2629–2646. https://doi.org/10.1080/00397911.2020.1783685
Pu Q, Kazemi M, Mohammadi M (2020) Application of transition metals in sulfoxidation reactions. Mini Rev Org Chem 17:423–449. https://doi.org/10.2174/1570193X16666190430154835
Mohamed Shams Y, Al Malak S (2023) Aromatic sulfonamides: S–N bond formation using MNPs-Benzo[d] imidazole-Cu magnetic catalyst. J Synth Chem 2:227–239. https://doi.org/10.22034/jsc.2023.430135.1058
Chen L, Noory Fajer A, Yessimbekov Z et al (2019) Diaryl sulfides synthesis: copper catalysts in C–S bond formation. J Sulfur Chem 40:451–468. https://doi.org/10.1080/17415993.2019.1596268
Mustafa AM (2023) Alum as a reusable catalyst for one-pot synthesis of acetylthiazoles under ultrasound irradiation in water. J Synth Chem 2:106–114. https://doi.org/10.22034/jsc.2023.176561
Shinde P, Rout CS (2021) Advances in synthesis, properties and emerging applications of tin sulfides and its heterostructures. Mater Chem Front 5:516–556. https://doi.org/10.1039/D0QM00470G
Yu X-Z, Wei W-L, Niu Y-L et al (2022) Homocouplings of sodium arenesulfinates: selective access to symmetric diaryl sulfides and diaryl disulfides. Molecules 27:6232. https://doi.org/10.3390/molecules27196232
Beletskaya IP, Ananikov VP (2022) Transition-metal-catalyzed C–S, C–Se, and C–Te bond formations via cross-coupling and atom-economic addition reactions. Achievements and challenges. Chem Rev. https://doi.org/10.1021/acs.chemrev.1c00836
Shibata T, Iwaki T, Ito M (2022) Ir-catalyzed intramolecular cyclization of 2-alkynyl diaryl sulfides for the selective synthesis of sulfur-containing polycyclic compounds. Adv Synth Catal 364:3472–3476. https://doi.org/10.1002/adsc.202200565
Zhou W-Y, Chen M, Zhang P-Z et al (2021) Methods, syntheses and characterization of diaryl, aryl benzyl, and dibenzyl sulfides. J Chem Crystallogr 51:301–310. https://doi.org/10.1007/s10870-020-00859-w
Roy P, Srivastava SK (2015) Nanostructured copper sulfides: synthesis, properties and applications. CrystEngComm 17:7801–7815. https://doi.org/10.1039/C5CE01304F
Eichman CC, Stambuli JP (2011) Transition metal catalyzed synthesis of aryl sulfides. Molecules 16:590–608. https://doi.org/10.3390/molecules16010590
Zhang R, Ding H, Pu X et al (2020) Recent advances in the synthesis of sulfides, sulfoxides and sulfones via C–S bond construction from non-halide substrates. Catalysts 10:1339. https://doi.org/10.3390/catal10111339
Sundaravelu N, Sangeetha S, Sekar G (2021) Metal-catalyzed C–S bond formation using sulfur surrogates. Org Biomol Chem 19:1459–1482. https://doi.org/10.1039/D0OB02320E
Li Y, Li X, Li X, Shi D (2021) Visible-light-promoted E-selective synthesis of α-fluoro-β-arylalkenyl sulfides via the deoxygenation/isomerization process. Chem Commun 57:2152–2155. https://doi.org/10.1039/D0CC08254F
Motahharifar N, Nasrollahzadeh M, Taheri-Kafrani A et al (2020) Magnetic chitosan–copper nanocomposite: a plant assembled catalyst for the synthesis of amino- and N-sulfonyl tetrazoles in eco-friendly media. Carbohydr Polym 232:115819. https://doi.org/10.1016/j.carbpol.2019.115819
Rezayati S, Ramazani A, Sajjadifar S et al (2021) Design of a Schiff base complex of copper coated on epoxy-modified core-shell MNPs as an environmentally friendly and novel catalyst for the one-pot synthesis of various chromene-annulated heterocycles. ACS Omega 6:25608–25622. https://doi.org/10.1021/acsomega.1c03672
Fang Y, Chen S, Chang L-Y (2024) Construction and characterization of a magnetic nanoparticle-supported Cu complex: a stable and active nanocatalyst for synthesis of heteroaryl-aryl and di-heteroaryl sulfides. RSC Adv 14:812–830. https://doi.org/10.1039/D3RA07791H
Guo Y-J, Lu S, Tian L-L et al (2018) Iodine-mediated difunctionalization of imidazopyridines with sodium sulfinates: synthesis of sulfones and sulfides. J Org Chem 83:338–349. https://doi.org/10.1021/acs.joc.7b02734
Gao Z, Zhu X, Zhang R (2014) Cs2CO3 promoted direct C–H bond sulfenylation of imidazo[1,2-a]pyridines and related heteroarenes in ionic liquid. RSC Adv 4:19891–19895. https://doi.org/10.1039/C4RA01240B
Le Bescont J, Breton-Patient C, Piguel S (2020) Unconventional reactivity with DABCO-Bis (sulfur dioxide): C–H bond sulfenylation of imidazopyridines. Eur J Org Chem 2020:2101–2109. https://doi.org/10.1002/ejoc.202000112
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, L., Cheng, Y., Ma, W. et al. Magnetic Nanoparticles Immobilized Copper(I) Complex: A Novel and Highly Active Catalyst for S-Arylation of Heterocycles. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04644-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04644-8