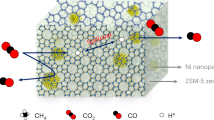
Abstract
Dry reforming of methane (DRM) has garnered significant attention because it is featured by the conversion of the two most representative greenhouse gases CH4 and CO2 into syngas composed of H2 and CO as target products. In our previous studies, we demonstrated the high catalytic activity and stability in DRM for the nickel silicate BEA-type zeolite (Ni-BEA) catalyst synthesized by interzeolite transformation. Here, the molybdenum modified Mo/Ni-BEA was prepared by deposition precipitation method on Ni-BEA, and its DRM activity was evaluated. Compared to the bare Ni-BEA, the modified Mo/Ni-BEA showed the more enhanced activity and stability with low carbon deposition, e.g., 9% lower coke content after 12 h on stream under harsh DRM reaction conditions, because of the promoter effect of molybdenum. Furthermore, Mo/Ni-BEA exhibited the stronger stability, i.e., both the deactivation rates for CH4 and CO2 were less than half compared to those of Ni-BEA, in a long-term DRM test for 55 h. To investigate the role of molybdenum as a promoter, the catalysts before and after reactions were analyzed through various characterization methods.
Graphical Abstract









Similar content being viewed by others
References
Usman M, Daud WMAW, Abbas HF (2015) Dry reforming of methane: influence of process parameters−a review. Renew Sust Energ Rev 45:710–744. https://doi.org/10.1016/j.rser.2015.02.026
Oyama ST, Hacarlioglu P, Gu Y, Lee D (2012) Dry reforming of methane has no future for hydrogen production: comparison with steam reforming at high pressure in standard and membrane reactors. Int J Hydrog Energy 37:10444–10450. https://doi.org/10.1016/j.ijhydene.2011.09.149
Wurzel T, Malcus S, Mleczko L (2000) Reaction engineering investigations of CO2 reforming in a fluidized-bed reactor. Chem Eng Sci 55:3955–3966. https://doi.org/10.1016/S0009-2509(99)00444-3
Er-rbib H, Bouallou C, Werkoff F (2012) Dry reforming of methane – review of feasibility studies. Chem Eng Trans 29:163–168. https://doi.org/10.3303/CET1229028
Pakhare D, Spivey J (2014) A review of dry (CO2) reforming of methane over noble metal catalysts. Chem Soc Rev 43:7813–7837. https://doi.org/10.1039/C3CS60395D
Xiancai L, Shuigen L, Yifeng Y, Min W, Fei H (2007) Studies on coke formation and coke species of nickel-based catalysts in CO2 reforming of CH4. Catal Lett 118:59–63. https://doi.org/10.1007/s10562-007-9140-7
Abdulrasheed A, Jalil AA, Gambo Y, Ibrahim M, Hambali HU, Hamid MYS (2019) A review on catalyst development for dry reforming of methane to syngas: recent advances. Renew Sust Energy Rev 108:175–193. https://doi.org/10.1016/j.rser.2019.03.054
Ranjekar AM, Yadav GD (2021) Dry reforming of methane for syngas production: a review and assessment of catalyst development and efficacy. J Indian Chem Soc 98:100002. https://doi.org/10.1016/j.jics.2021.100002
Zhou R, Mahinpey N (2023) A review on catalyst development for conventional thermal dry reforming of methane at low temperature. Can J Chem Eng 101:3180–3212. https://doi.org/10.1002/cjce.24876
Abdullah B, Ghani NAA, Vo DVN (2017) Recent advances in dry reforming of methane over Ni-based catalysts. J Clean Prod 162:170–185. https://doi.org/10.1016/j.jclepro.2017.05.176
Dekkar S, Tezkratt S, Sellam D, Ikkour K, Parkhomenko K, Martinez-Martin A, Roger AC (2020) Dry reforming of methane over Ni–Al2O3 and Ni–SiO2 catalysts: role of preparation methods. Catal Lett 150:2180–2199. https://doi.org/10.1007/s10562-020-03120-3
Hammond C, Padovan D, Tarantino G (2018) Porous metallosilicates for heterogeneous, liquid-phase catalysis: perspectives and pertaining challenges. R Soc Open Sci 5:171315. https://doi.org/10.1098/rsos.171315
Kweon S, Kim YW, Shin CH, Park MB, Min HK (2022) Nickel silicate beta zeolite prepared by interzeolite transformation: a highly active and stable catalyst for dry reforming of methane. Chem Eng J 431:133364. https://doi.org/10.1016/j.cej.2021.133364
Kweon S, Bae J, Cho Y, Lee S, Kim J, Jo D, Shin CH, Park MB, Min HK (2022) Defect-stabilized nickel on beta zeolite as a promising catalyst for dry reforming of methane. Catal Sci Technol 12:3106–3115. https://doi.org/10.1039/D1CY02363B
Yao L, Zhu J, Peng X, Tong D, Hu C (2013) Comparative study on the promotion effect of Mn and Zr on the stability of Ni/SiO2 catalyst for CO2 reforming of methane. Int J Hydrog Energy 38:7268–7279. https://doi.org/10.1016/j.ijhydene.2013.02.126
Li L, Anjum DH, Zhu H, Saih Y, Laveille PV, D’Souza L, Basset JM (2015) Synergetic effects leading to coke-resistant NiCo bimetallic catalysts for dry reforming of methane. ChemCatChem 7:427–433. https://doi.org/10.1002/cctc.201402921
Huang T, Huang W, Huang J, Ji P (2011) Methane reforming reaction with carbon dioxide over SBA-15 supported Ni–Mo bimetallic catalysts. Fuel Process Technol 92:1868–1875. https://doi.org/10.1016/j.fuproc.2011.05.002
Yao L, Galvez ME, Hu C, Costa PD (2017) Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int J Hydrog Energy 42:23500–23507. https://doi.org/10.1016/j.ijhydene.2017.03.208
Xiao T, Suhartanto T, York APE, Sloan J, Green MLH (2003) Effect of molybdenum additives on the performance of supported nickel catalysts for methane dry reforming. Appl Catal A: Gen 253:225–235. https://doi.org/10.1016/S0926-860X(03)00522-2
Quincoces CE, de Vargas SP, Grange P, González MG (2002) Role of Mo in CO2 reforming of CH4 over Mo promoted Ni/Al2O3 catalysts. Mater Lett 56:698–704. https://doi.org/10.1016/S0167-577X(02)00598-0
Millini R, Perego G, Parker WO, Bellussi G, Carluccio L (1995) Layered structure of ERB-1 microporous borosilicate precursor and its intercalation properties towards polar molecules. Microporous Mater 4:221–230. https://doi.org/10.1016/0927-6513(95)00013-Y
de Jong KP (2009) Synthesis of solid catalysts. Wiley-VCH, Germany
Srivastava N, Srivastava PC (2010) Realizing NiO nanocrystals from a simple chemical method. Bull Mater Sci 33:653–656. https://doi.org/10.1007/s12034-011-0142-0
Jia X, Lin Z, Yang TCJ, Puthen-Veettil B, Wu L, Conibeer G, Perez-Wurfl I (2018) Post-sputtering heat treatments of molybdenum on silicon wafer. Appl Sci 8:1692. https://doi.org/10.3390/app8091692
Reddy RKK, Kailasa S, Rani BG, Jayarambabu N, Yasuhiko H, Ramana GV, Rao KV (2019) Hydrothermal approached 1-D molybdenum oxide nanostructures for high-performance supercapacitor application. SN Appl Sci 1:1365. https://doi.org/10.1007/s42452-019-1295-5
Qi Y, Qi H, Li J, Lu C (2008) Synthesis, microstructures and UV–vis absorption properties of β-Ni(OH)2 nanoplates and NiO nanostructures. J Cryst Growth 310:4221–4225. https://doi.org/10.1016/j.jcrysgro.2008.06.047
Dhas NA, Gedanken A (1997) Sonochemical synthesis of molybdenum oxide− and molybdenum carbide−silica nanocomposites. Chem Mater 9:3144–3154. https://doi.org/10.1021/cm9704488
Li J, Liu H, An T, Yueb Y, Bao X (2017) Carboxylic acids to butyl esters over dealuminated–realuminated beta zeolites for removing organic acids from bio-oils. RSC Adv 7:33714–33725. https://doi.org/10.1039/C7RA05298G
Yang C, Xuaf Q (1997) States of aluminum in zeolite β and influence of acidic or basic medium. Zeolites 19:404–410. https://doi.org/10.1016/S0144-2449(97)00103-6
Min HK, Kweon S, Kim YW, An H, Jo D, Park ED, Shin CH, Park MB (2021) Atomically dispersed nickel species in a two-dimensional molecular sieve: origin of high activity and stability in dry reforming of methane. Appl Catal B: Environ 298:120627. https://doi.org/10.1016/j.apcatb.2021.120627
He T, Yao J (2003) Photochromism of molybdenum oxide. J Photochem Photobiol C 4:125–143. https://doi.org/10.1016/S1389-5567(03)00025-X
Aniwar H, Hogarth CA, Theocharis CR (1989) A study of the infrared absorption spectra of thin amorphous films of molybdenum trioxide. J Mater Sci 24:2387–2390. https://doi.org/10.1007/BF01174500
Tsai Y-C, Wu M-S (2020) Zeolitic nickel phosphate nanorods with open-framework structure (VSB-5) for catalytic application in electro-oxidation of urea. Appl Surf Sci 529:147175. https://doi.org/10.1016/j.apsusc.2020.147175
Davidson A, Tempere JF, Che M, Roulet H, Dufour G (1996) Spectroscopic studies of Nickel(II) and Nickel(III) species generated upon thermal treatments of Nickel/Ceria-supported materials. J Phys Chem 100:4919–4929. https://doi.org/10.1021/jp952268w
Lehmann T, Wolff T, Hamel C, Veit P, Garke B, Seidel-Morgenstern A (2012) Physico-chemical characterization of Ni/MCM-41 synthesized by a template ion exchange approach. Microporous Mesoporous Mater 151:113–125. https://doi.org/10.1016/j.micromeso.2011.11.006
Yan L, Liu X, Deng J, Fu Y (2017) Molybdenum modified nickel phyllosilicates as a high performance bifunctional catalyst for deoxygenation of methyl palmitate to alkanes under mild conditions. Green Chem 19:4600–4699. https://doi.org/10.1039/C7GC01720K
Xiang J, Yan Z (2020) Experimental study on tribological characteristics in coke powder lubrication. Adv Mech Eng. https://doi.org/10.1177/1687814020940454
Acknowledgements
This work was supported by the Incheon National University Research Grant in 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S., Kweon, S. & Park, M.B. Molybdenum Promoted Nickel Silicate BEA-Type Zeolite Catalyst for Dry Reforming of Methane. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04605-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04605-1