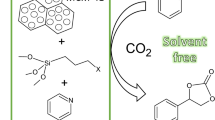
Abstract
Novel imidazolinium based materials were prepared by modification of MCM-41 and characterized by elemental analysis, nitrogen sorption, thermogravimetric analysis, UV–Vis, and X‑ray diffraction. The materials differed in the alkyl, or halide used (MeI, EtBr, PrI, BuBr, BuCl, and BnBr). The characterization methods confirmed the successful formation of desired materials containing iodide and bromide. In this research, the materials were examined as catalysts for the cycloaddition of carbon dioxide to styrene oxide to produce styrene carbonate. The influence of anion type and the length of the alkyl chain in the salt was discussed. The results show imidazolinium materials as efficient heterogeneous catalysts for the model reaction. Styrene carbonate was prepared with high selectivities in all cases. The highest conversion of styrene oxide (95%) was achieved using a material containing iodomethane (MCM-Im-MeI) under the following conditions: 1.2 MPa, 120 °C, solvent free. The catalysts were successfully reused without a significant decrease of their activity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cyclic carbonates are compounds with high boiling points, low toxicity, and often biodegradability. They find application in many fields of the chemical industry. They are applied as aprotic solvents, electrolytes in lithium cells, and intermediates for the production of pesticides and pharmaceuticals. Their utilization as a part of oil and colorants, or monomers for the production of polycarbonates or polyurethanes is also very important [1,2,3], especially by avoiding the use of phosgene. The most often and most effective process for the production of cyclic carbonates (even on the industrial level) is the cycloaddition reaction of carbon dioxide to corresponding epoxides. Such a process takes advantage even from the consumption of available and partly problematic carbon dioxide and may be included in the group of processes decreasing the concentration of this gas [4]. The most often offered mechanism [4,5,6] for the production of cyclic carbonates is started by epoxide activation. This step needs an acidic catalyst, either Lewis or Brønsted type. Metal base catalysts react as Lewis acid, organocatalysts as Brønsted acid. The second step is the epoxide opening under nucleophile attack. Nucleophile, which is also leaving group is almost always halide (Cl−, Br−, I−). Halide may be a part of a catalyst or may be added to the reaction mixture, mostly in the form of tetraalkylammonium salt. Quaternary ammonium salts themselves may be used also as catalysts without any additive. The most used catalyst from this group for cycloaddition reaction of carbon dioxide and epoxide is tetrabutylammonium bromide (TBAB), e.g., [7]. However, some quaternary ammonium salts possess other interesting properties that may be beneficial in the discussed application. Imidazolium salts may behave as ionic liquids, thus substituted variants of imidazolium ionic liquids were used in the preparation of cyclic carbonates [8]. 3-Benzyl-1-methylimidazolium salt was synthesized without any solvent and yields up to 96% of cyclic carbonates were obtained. Aminofunctionalized imidazolium ionic liquids were used [9] in the reaction of carbon dioxide with epichlorohydrin. The reaction was performed without any solvent and the yield was up to 99%.
To enable the separation of catalysts from the reaction mixture and simplify the process of their reuse, the catalysts immobilized on the solid support are prepared. The most used materials for supporting the catalysts (especially in the field of cyclic carbonates production, [10, 11]) are silica based materials with the defined mesoporous structure, e.g., MCM-41 and SBA-15. SBA-15 modified by triethanolamine was used for the cycloaddition of CO2 to propylene oxide [12]. The yield was 94% (2 MPa and 110 °C). The catalyst was separated by filtration and reused five times without loss of activity. The same support together with MCM-41 was used for the anchorage of tripropylammonium iodide [13]. The prepared material was used for the production of styrene carbonate from styrene and carbon dioxide. The yields up to 99% (using MCM-41) under 0.1 MPa and 25 °C were obtained. Modified MCM-41 was reused four times without loss of activity; the use of SBA-15 was less successful. Bifunctional phosphonium and ammonium salts immobilized on polystyrene were used for the addition of CO2 to glycidyl methacrylate [14]. The conversions were 95–99% under 90 °C and 1 MPa. Catalyst was reused 12 times with a loss of yield; however, the final yield was still higher than 60%. In our previous work [15], we designed and tested new catalysts based on pyridinium salts immobilized on MCM-41. Using such catalysts (1.2 MPa and 120 °C) yield of styrene carbonate from styrene oxide and carbon dioxide was up to 99% dependently on anion (Cl, Br, I) and used solvent. The type of anion and solvent also influenced the reusability of the catalyst.
In this work, we want to show the efficient use of heterogeneously anchored imidazolinium salt in a model reaction of styrene oxide with carbon dioxide to styrene carbonate. The influence of anion type and also the length of the alkyl chain in the salt was also discussed.
2 Experimental
2.1 Materials
Triethoxy-3-(2-imidazolin-1-yl)propylsilane (> 97%), 1-chlorobutane (99.5%), 1-bromobutane (99%), benzyl bromide (98%), bromoethane (98%), iodomethane (> 99%), 1-iodopropane (99%), hexadecyltrimethylammonium bromide (> 98%) and tetraethoxysilane (98%) were purchased from Sigma-Aldrich. Ammonia (26% aqueous solution) and N,N-dimethylformamide (p.a.) were obtained from Penta. Styrene oxide (> 98%) was purchased from TCI and toluene (p.a.) was obtained from Lach:ner. Demineralized water was taken from UCT Prague sources (< 1 μS/mL). All chemicals except toluene were used without further purification. Toluene was distilled over sodium prior to use.
2.2 Catalysts Preparation
2.2.1 Preparation of MCM-41
Hexadecyltrimethylammonium bromide (2.4 g) was dissolved in demineralized water (120 mL) preheated to 35 °C. After dissolution, an aqueous ammonia solution (26%, 10 mL) was added. Then 10 mL of tetraethoxysilane was added. The suspension was subsequently stirred for at least 18 h at room temperature and after that filtered. The solid was washed with demineralized water and ethanol (3 × 10 mL) and dried at 100 °C for 1 h. The obtained product was calcined at 550 °C for 2 h under a nitrogen atmosphere and then in the air overnight.
The resulting MCM-41 was subsequently stirred in demineralized water for 24 h in order to increase the amount of hydroxyl groups. MCM-41 was filtered and dried at 100 °C.
2.2.2 Modification of MCM-41
4 mmol of triethoxy-3-(2-imidazolin-1-yl)propylsilane were dissolved in 20 mL of toluene. 1 g of MCM-41 was added to the solution and the suspension was stirred under reflux (120 °C) for 5 h. Finally, the resulting solid was filtered, dried overnight at 100 °C, and denoted as MCM-Im.
The dried MCM-Im (0.8 g) was suspended in 10 mL of toluene and 3.2 mmol of alkyl halide was added. 0.8 g of MCM-Im contained 1.6 mmol of triethoxy-3-(2-imidazolin-1-yl)propylsilane which corresponded to 0.4 mmol of 2-imidazoline. The mixture was stirred under reflux (120 °C). After 24 h, the prepared material was filtered and dried at 100 °C overnight. Dependently on the used alkyl halide the materials were denoted as MCM-Im-RX, where R represents the alkyl part (Me-methyl, Et-ethyl, Pr-propyl, Bu-butyl, Bn-benzyl) and X halogen (I, Br, Cl).
2.3 Catalyst Testing
Experiments were performed in a stainless-steel autoclave Parr 4842 (volume 50 mL) (Parr Instrument Company, US). 1.2 g (10 mmol) of styrene oxide dissolved in toluene or DMF and 300 mg of solid catalyst were dosed to the reactor. In the solvent free arrangement, 300 mg of solid catalyst were dosed to the reactor together with styrene oxide (10 mmol). The autoclave was closed, washed with carbon dioxide, and heated to 120 °C. After reaching the temperature, the pressure was increased by carbon dioxide to 1.2 MPa and the reaction started. Samples from the reaction mixture were centrifuged and analyzed by GC Shimadzu 2010 (FID, non-polar column ZB-5, Shimadzu, Japan). For the structure confirmation, the GC–MS Shimadzu 2010 (non-polar column DB-5 ms, quadrupole detector) was used.
2.4 Catalyst Characterization
The elemental composition of prepared catalysts was measured by elemental analysis (EA). Elemental analysis (C,H,N) was performed using Elementar Vario EL Cube (Elementar, Germany). The accuracy of the method was determined by the manufacturer for the simultaneous analysis of 5 mg of the standard 4-amino-benzene sulfonic acid in the CHNS module to < 0.1% abs. for each element. The halogen content (I, Br, Cl) was determined by the classic argentometric titration analysis modified for low sample loading. Thermogravimetric analysis (TG) was performed from 30 to 550 °C with the step 10 °C/min under nitrogen atmosphere using Setaram Setsys Evolution equipment (Setaram, France). Nitrogen sorption was measured at Micromeritics ASAP 2020 V300 H (Micromeritics). For the calculation of specific surfaces and pore volume, BET/BJH equations were used. DRUV/VIS of solid samples was performed on Shimadzu UV2600i using an integrating sphere (Shimadzu, Japan). Samples were diluted by BaSO4 and measured in a quartz cell with the slit 5 nm. X‑ray diffraction (XRD) in the low angle mode was used to confirm the MCM-41 structure. The used equipment was PANalytical X´Pert PRO (PANalytical, UK).
3 Results and Discussion
In this work, the supported imidazolinium salts were prepared and tested as catalysts in the cycloaddition of carbon dioxide to styrene oxide. These materials were prepared by modification of support material, MCM-41, by triethoxy-3-(2-imidazolin-1-yl)propylsilane. The nitrogen atom on N-heterocycle was subsequently quaternized by alkyl halide (Fig. 1). More probable was the quaternization of one nitrogen (Fig. 1a) due to steric reasons. Nevertheless, quaternization of both nitrogen atoms was possible (Fig. 1b), and would lead to the formation of moiety also active in the cycloaddition reaction.
3.1 Catalyst Characterization
Elemental analysis (Table 1) confirmed the presence and the amount of attached imidazoline derivative on the support and the presence and amount of halogen in the structure after quaternization.
We can say that the surface modification of MCM-41 was successful. The amount of attached imidazoline was comparable in all cases (15–17 wt. % on MCM-41). At the same time, we observed that the amount of attached halogen decreased in the row: I > Br > Cl. However, taking into account the molecular weights of different halogens, the amount of quaternized salts ranged from 21% (Im-EtBr) to 27% (Im-PrI and Im-BnBr). The only exception was chloride. Using BuCl the quaternization was not really successful. These results correspond with our previous study [15]. The degree of quaternization (\(DQ= \frac{n\left(X\right)*2}{n(N)}\)) was calculated (Table 1), from which we could say that this parameter was comparable in all cases (except the material containing chlorine in its structure) and was up to 70%. This value means that 30% of imidazoline molecules remained nonquaternized. However, it is impossible to decide whether imidazoline molecules had only one or both nitrogen atoms quaternized, and which one was quaternized. However, the more probable was the quaternization of only one nitrogen atom due to an electrostatic repulsion if two quaternary salts were formed. The nitrogen atom bearing double bond was less steric hindered and was more easily accessible for quaternization. However, at the same time, this nitrogen was less nucleophilic, and thus quaternization on the second, more nucleophilic, nitrogen atom was assumed.
XRD measurement confirmed the preservation of MCM-41 structure even after modification by alkyl halides. The visible difference was in intensity. The intensity of characteristic bands decreased after the modification (Fig. S1).
Thermogravimetric analysis confirmed the stability of prepared materials up to 150 °C. Above this temperature, the decrease of weight in all cases was observed. The most gentle weight decrease was detected in the case of the material without quaternization (MCM-Im) as we expected. Imidazoline was attached to the surface of MCM-41 by a covalent bond through a propyl linker. TG confirmed (Fig. 2) that all quaternization agents were attached to imidazolinium by stronger interaction than nonbonding because no weight decrease was observed around any boiling point of quaternization agents (e.g., EtBr 38 °C, MeI 42 °C). The total loss of weight depended mainly on the halogen present in the material and thus was higher for iodide based catalysts (weight loss at 550 °C increased in the row: MCM-BnBr < MCM-EtBr < MCM-BuBr < MCM-PrI ≡ MCM-MeI). Almost all materials modified with halogens possessed a similar TG profile and had the steepest weight decrease at approx. 390 °C. This has to be connected with the decomposition of the imidazolinium unit connected to MCM-41 because each salt has different thermal stability as described, e.g., in [16]. MCM-Im-MeI possessed a different profile, probably due to the size of methyl, which was the smallest of all alkyls used. The size of the methyl group could enable the attachment to both nitrogen atoms of imidazoline (Fig. 1b). We also want to explain a different profile of MCM-Im-MeI by the presence of different interactions. Imidazoline was in this case attached firmly (cationic form, decrease at 390 °C), as in the other materials, and weakly—probably dication, the decrease 200–330 °C. This was also confirmed by UV/Vis. Other alkyls were probably attached predominantly to the less sterically hindered nitrogen atom.
The comparison of UV–Vis spectra of MCM-Im and quaternized materials is shown in Fig. 3. Imidazoline modified MCM-41 in the measured region 220–550 nm possessed by one band at approx. 230 nm assignable to imidazoline. Quaternized materials except MCM-Im-MeI possessed by a similar band slightly shifted to higher wavelengths to approx. 240 nm with a significant maximum. This might be connected with the formation of imidazolinium ion by quaternization. Shift dependent on neither alkyl group nor anion was observed and it was in correlation with literary data [17]. To our best knowledge, we have found no evidence about UV–Vis spectra of imidazolinium salts, but similar behavior might be expected for imidazolium and even pyridinium salts. Thus we suppose that band at 240 nm might be assignable to π–π* excitation connected with trivalent nitrogen [18]. The intensity of the band was in correlation with TGA data and might be attributed to the used halogen. The most interesting was the spectra of MCM-Im-MeI. It showed three bands with a maximum at 360, 295, and 230 nm. Based on pyridine behavior [15], we tried to assign these bands to a charge transfer between imidazoline and isolated silanol groups, π–π* excitation (imidazoline) and π–π* excitation (imidazolinium), respectively [18]. The difference between MCM-Im-MeI and others might be explained by the size of the methyl group that enables also the formation of dication from both nitrogens present in the molecule.
The surface characteristics significantly changed after the modification of MCM-41 (Table 2). The specific surface area decreased even after the modification imidazoline modifier to 10% of its original value and obviously also decreased after the modification by alkyl halides. This was connected with the decrease of pore volume. The average pore size was influenced by the blockage of mesopores of MCM‑41 by the modifier. The results are in the correlation with the data obtained from XRD.
From Fig. 4 the missing increase/decrease in the range of relative pressures 0.2–0.3 is obvious. This change is typical for MCM-41 and is attributed to capillary condensation in uniform open mesopores (2–3 nm). As we know that MCM-41 was used as starting material and its isotherm was typical (Fig. S2 in SI) we can say that these typical pores were blocked even by propyl imidazole (MCM-Im). In the range of relative pressures 0.3–0.9, the nitrogen adsorption occurred only on the external surface (minimal increase of adsorbed volume). The final increase of adsorbed nitrogen above value of relative pressure 0.9 probably corresponded to capillary condensation in space between particles. Very narrow hysteresis showed the pores open only on one side; more over the capillary condensation was not in the pores but on the outer surface. MCM-Im containing only propyl imidazoline blocked the pores the least of all materials (accompanied by the highest specific surface). The highest decrease of the adsorbed amount and with it connected lower specific surface is attributed to the presence of alkyls and halides. It was obvious that materials with smaller Br molecule (MCM-Im-EtBr, MCM-Im-BuBr) adsorbed higher amounts of nitrogen compared to materials with larger I molecule (MCM-Im-MeI, MCM-Im-PrI). In the case of the latter mentioned materials, also the decrease of specific surface and adsorbed amount with increasing size of alkyl was visible. The lowest adsorbed amount of nitrogen showed the material with benzyl in the structure—MCM-Im-BnBr. This was caused by the presence of the largest substituent. Distribution of mesopore volume of prepared samples was calculated using BJH and DFT methods. We can say that the materials were mesoporous in the range of very small mesopores and their blockage was obvious. The pore size distribution was narrow for all modified materials (Fig. S3 in SI).
3.2 Catalytic Tests
The catalytic activity of prepared materials was tested in a model reaction, cycloaddition of carbon dioxide to styrene oxide producing styrene carbonate (Fig. 5). The influence of the catalyst structure on its catalytic activity was studied as the main part of our work. This issue was divided into two parts: the influence of halide type and the influence of the alkyl chain.
Three halides (I, Br, Cl) were used for the testing of their influence on the reaction course. To eliminate the influence of the alkyl chain similar materials were used—MCM-Im-PrI, MCM-Im-BuBr, and MCM-Im-BuCl. The activity of the last mentioned was negligible (Fig. 6), which corresponded with the low degree of quaternization. The molar ratio styrene oxide:halogen was 35:1 in all cases except material containing chloride. Because of the low degree of quaternization the molar ratio styrene oxide:chloride was 195:1. The amount of iodide and bromide in the materials was comparable as same as the quaternization degree, thus we can say that more active was the catalyst containing iodide in its structure. The higher activity of the material containing iodide was in the correlation with the nucleophilicity of halogen ions, which decreased in the row I > Br > Cl. This result corresponded with the results from the literature [19]. However, the role might also play a slightly different alkyl group present in the material.
The effect of alkyl chains in the catalysts on the reaction course can be categorized into two groups based on the anion present in the catalyst structure. The first group consisted of catalysts containing iodide in their structure, such as MCM-Im-MeI and MCM-Im-PrI. The second group comprised catalysts containing bromide, such as MCM-Im-EtBr, MCM-Im-BuBr, and MCM-Im-BnBr. In both cases, there was a consistent trend: as the length of the alkyl chain increased, the conversion of styrene oxide decreased (Fig. 7).
Among the catalysts with iodine, MCM-Im-MeI, which has the smallest alkyl group, enabled the highest conversion. For MCM-Im-PrI and MCM-Im-Et-Br, the conversions were comparable, indicating that the positive influence of shorter chain length (ethyl) on conversion was counteracted by the nucleophilicity of the halogen (bromine) and vice versa: the positive influence of the halogen (iodine) and longer chain (propyl).
The catalysts MCM-Im-BuBr and MCM-Im-BnBr followed the trend mentioned above; using these catalysts the conversion of styrene oxide was the lowest. In particular, the material containing the benzyl group offered very low conversion of SO (only 7% after 300 min). The negative effect of the benzyl group could be attributed to its size and steric hindrance, which aligned with its lowest specific surface area.
Two solvents were tested in the studied reaction using two different materials (MCM-Im-MeI and MCM-Im-EtBr, Table 3). We found that using toluene the lower conversions were achieved comparing the reactions using DMF as a solvent in the case of both used catalysts. Moreover, the ratio between conversion in toluene and DMF differed based on the used catalyst: for MCM-Im-MeI the ratio Xtoluene:XDMF was 0.67 whilst for MCM-Im-EtBr was 0.43. The explanation probably lies in the polarity of used solvents. DMF as a polar solvent promote the reaction while toluene as a non-polar solvent does not favor the reaction. However, toluene can interact with imidazolinium (C-H…π bond) [20], which can provide stabilization of the imidazolinium moiety. The lower selectivity using DMF as a solvent in both cases may be explained by the formation of 1-phenyl-1,2-ethandiole as a by-product. This compound was formed in the presence of water in the reaction mixture.
The solvent free arrangement usually yielded higher conversions that are given by the direct contact of the reagent and carbon dioxide. Thus, the solvent free arrangement was carried out. The reaction mixture consisted of styrene oxide, catalyst and carbon dioxide under the same pressure and temperature as reactions with solvent. The limited solubility of carbon dioxide in different solvents always decreased the reaction rate (conversion). We have already described this phenomenon in our previous work [15]. This was also confirmed in the studied reactions using materials MCM-Im-MeI and MCM-Im-EtBr (Table 3). In both cases, a higher conversion of styrene oxide was observed in the solvent free arrangement.
The main advantage of a heterogeneous catalyst is its easy separation from the reaction mixture and possible reuse. The most efficient catalyst MCM-Im-MeI was filtered from the reaction mixture, washed with toluene, dried (100 °C, overnight), and reused. The obtained results showed no decrease of selectivity and only a slight decrease of conversion after the second use. This implies that the catalyst is promising for repeated use in large-scale reactions. A comparison of the catalytic activity of prepared materials with various heterogeneous catalysts described in the literature was performed (Table 4). Conversions of styrene oxide are comparable with published data.
4 Conclusion
New MCM-41 supported imidazolinium salts were prepared for use in a cycloaddition of carbon dioxide to styrene oxide. These materials were prepared in two steps: first, the modification of the support material by imidazolinyl silane linker, followed by the reaction with different quaternization agents, alkyl halides. Prepared materials were characterized by available methods—EA, XRD, TG, UV–Vis, and nitrogen sorption. Elemental analysis confirmed the presence and the amount of attached imidazoline derivative and halogen after quaternization. The amount of attached imidazoline was comparable in all cases. XRD confirmed the preservation of MCM‑41 structure after the modifications. Nitrogen sorption provided information on the specific surface of the material. Thermogravimetric analysis showed that imidazoline was attached to the surface of MCM-41 by a covalent bond through a propyl linker. TG spectra of MCM-Im-MeI showed that the methyl group is able to attach to both nitrogen atoms of imidazoline derivative. This was confirmed also by UV–Vis. In general, it can be said that characterization methods confirmed the successful preparation of iodide and bromide based catalysts. The preparation of chloride based catalyst was unsuccessful (low quaternization degree). Catalytic activity was verified in a model reaction of carbon dioxide with styrene oxide producing styrene carbonate. The influence of anion type and also the length of the alkyl chain in the salt was discussed. The activity of the catalyst depended on the nucleophilicity of halogen ions, which decreases in the row: I > Br > Cl. Moreover, both bromide and iodide catalysts confirmed the same trend: the conversion of styrene oxide decreased with the increase of the length of the alkyl chain. This trend can be explained by the steric hindrance of larger chains. Desired styrene carbonate was prepared with high selectivity in all cases. A solvent free rearrangement of the model reaction was also possible using prepared materials. The most active catalyst, MCM-Im-MeI, was successfully reused without a significant decrease of its activity. The prepared imidazolinium based salts showed results comparable to the catalysts previously described in the literature, and thus offer a new type of catalyst in this important reaction.
References
Calabrese C, Giacalone F, Aprile C (2019) Hybrid catalysts for CO2 conversion into cyclic carbonates. Catalysts 9:1–30. https://doi.org/10.3390/catal9040325
Sun J, Fujita S-I, Zhao F, Arai M (2004) Synthesis of styrene carbonate from styrene oxide and carbon dioxide in the presence of zinc bromide and ionic liquid under mild conditions. Green Chem 6:613–616. https://doi.org/10.1039/B413229G
Fang J, Li K, Wang Z, Li D, Ma Y, Gong X, Hou Z (2020) Direct oxidative carboxylation of olefins into cyclic carbonates at ambient pressure. J CO2 Util 40:101204. https://doi.org/10.1016/j.jcou.2020.101204
Pescarmona PP (2021) Cyclic carbonates synthesised from CO2: Applications, challenges and recent research trends. Curr Opin Green Sustain Chem 29:100457. https://doi.org/10.1016/j.cogsc.2021.100457
Zhang Z, Pan S-Y, Li H, Cai J, Olabi AG, Anthony EJ, Manovic V (2020) Recent advances in carbon dioxide utilization. Renew Sust Energy Rev 125:109799. https://doi.org/10.1016/j.rser.2020.109799
Guo L, Lamb KJ, North M (2021) Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem 23:77–118. https://doi.org/10.1039/D0GC03465G
Rehman A, Eze VC, Resul MFMG, Harvey A (2019) A kinetic study of Zn halide/TBAB-catalysed fixation of CO2 with styrene oxide in propylene carbonate. Green Process Synth 8:719–729. https://doi.org/10.1515/gps-2019-0042
Wang T, Zheng D, Ma Y, Guo J, He Z, Ma B, Liu L, Ren T, Wang L, Zhang J (2017) Benzyl substituted imidazolium ionic liquids as efficient solvent-free catalysts for the cycloaddition of CO2 with epoxides: Experimental and theoretic study. J CO2 Util 22:44–52. https://doi.org/10.1016/j.jcou.2017.09.009
Yue S, Song Q, Zang S, Deng G, Li J (2018) Amino-functional ionic liquids as efficient catalysts for the cycloaddition of carbon dioxide to yield cyclic carbonates: Catalytic and kinetic investigation. Aust J Chem 71:407–415. https://doi.org/10.1071/CH17656
Kim D-W, Roshan R, Tharun J, Cherian A, Park D-W (2013) Catalytic applications of immobilized ionic liquids for synthesis of cyclic carbonates from carbon dioxide and epoxides. Korean J Chem Eng 30:1973–1984. https://doi.org/10.1007/s11814-013-0193-6
Steinbauer J, Longwitz L, Frank M, Epping J, Kragld U, Werner T (2017) Immobilized bifunctional phosphonium salts as recyclable organocatalysts in the cycloaddition of CO2 and epoxides. Green Chem 19:4435–4445. https://doi.org/10.1039/C7GC01782K
Zhang M, Chu B, Li G, Xiao J, Zhang H, Peng Y, Li B, Xie P, Fan M, Dong L (2019) Triethanolamine-modified mesoporous SBA-15: Facile one-pot synthesis and its catalytic application for cycloaddition of CO2 with epoxides under mild conditions. Microporous Mesoporous Mater 274:363–372. https://doi.org/10.1016/j.micromeso.2018.09.011
Zakharova MV, Kleitz F, Fontaine F-G (2017) Carbon dioxide oversolubility in nanoconfined liquids for the synthesis of cyclic carbonates. ChemCatChem 9:1886–1890. https://doi.org/10.1002/cctc.201700247
Büttner H, Kohrt C, Wulf C, Schäffner B, Groenke K, Hu Y, Kruse D, Werner T (2019) Life cycle assessment for the organocatalytic synthesis of glycerol carbonate methacrylate. Chemsuschem 12:2701–2707. https://doi.org/10.1002/cssc.201900678
Vyskočilová E, Šafařík D, Zítová K, Vrbková E, Dimitrov R, Vagenknechtová A, Červený L (2022) Efficient catalyst based on pyridinium modified MCM-41 for carbon dioxide utilization. Catal Lett 152:3576–3585. https://doi.org/10.1007/s10562-022-03928-1
Awad WH, Gilman JW, Nyden M, Harris RH Jr, Sutto TE, Callahan J, Trulove PC, DeLong HC, Fox DM (2004) Thermal degradation studies of alkyl-imidazolium salts and their application in nanocomposites. Thermochim Acta 409:3–11. https://doi.org/10.1016/S0040-6031(03)00334-4
Tanabe I, Kurawaki Y, Morisawa Y, Ozaki Y (2016) Electronic absorption spectra of imidazolium-based ionic liquids studied by far-ultraviolet spectroscopy and quantum chemical calculations. Phys Chem Chem Phys 18:22526–22530. https://doi.org/10.1039/C6CP02930B
Velthoen MEZ, Nab S, Weckhuysen BM (2018) Probing acid sites in solid catalysts with pyridine UV-Vis spectroscopy. Phys Chem Chem Phys 20:21647–21659. https://doi.org/10.1039/C8CP03991G
Liu M, Liang L, Liang T, Lin X, Shi L, Wang F, Sun J (2015) Cycloaddition of CO2 and epoxides catalyzed by dicationic ionic liquids mediated metal halide: Influence of the dication on catalytic activity. J Mol Catal A Chem 408:242–249. https://doi.org/10.1016/j.molcata.2015.07.032
Chen H, Wang Z, Xu X, Gong S, Zhou Y (2021) The molecular behavior of pyridinium/imidazolium based ionic liquids and toluene binary systems. Phys Chem Chem Phys 23:13300. https://doi.org/10.1039/d1cp00874a
Ra CS, Hwang JC, Lee HB, Shim J-J (2007) Solvent-free carboxylation of styrene oxide: enhanced reactivity of quaternary onium salts in ionic liquid-CO2 system. Bull Korean Chem Soc 28:1060–1062. https://doi.org/10.5012/bkcs.2007.28.6.1060
Appaturi JN, Adam F (2013) A facile and efficient synthesis of styrene carbonate via cycloaddition of CO2 to styrene oxide over ordered mesoporous MCM-41-Imi/Br catalyst. Appl Catal B 136–137:150–159. https://doi.org/10.1016/j.apcatb.2013.01.049
Ghazali-Esfahani S, Song H, Paunescu E, Bobbink FD, Liu H, Fei Z, Laurenczy G, Bagherzadeh M, Yan N, Dyson PJ (2013) Cycloaddition of CO2 to epoxides catalyzed by imidazolium-based polymeric ionic liquids. Green Chem 15:1584–1589. https://doi.org/10.1039/C3GC37085B
Barbarini A, Maggi R, Mazzacani A, Mori G, Sartori G, Sartorio R (2003) Cycloaddition of CO2 to epoxides over both homogeneous and silica-supported guanidine catalysts. Tetrahedron Lett 44:2931–2934. https://doi.org/10.1016/S0040-4039(03)00424-6
Motokura K, Itagaki S, Iwasawa Y, Miyaji A, Baba T (2014) Zinc-accelerated cycloaddition of carbon dioxide to styrene oxide catalyzed by pyrrolidinopyridinium iodides. Top Catal 57:953–959. https://doi.org/10.1007/s11244-014-0257-9
Funding
Open access publishing supported by the National Technical Library in Prague. This work was supported from Grant Project GACR 21-02183S and the grant of Specific university research—Grant No. A1_FCHT_2023_001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zítová, K., Hudcová, M., Lhotka, M. et al. Imidazolinium Salts for the Carbonates Production from CO2 and Epoxides. Catal Lett 154, 1622–1630 (2024). https://doi.org/10.1007/s10562-023-04445-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04445-5