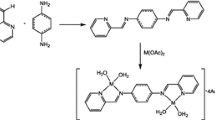
Abstract
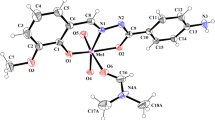
In this work, we reported the synthesis of ruthenium-p-cymene-Schiff base complexes (KB-1, KB-2) containing Schiff base ligands with the transfer hydrogenation (TH) of a series of ketones. The KB-1 and KB-2 complexes were characterized and used by different spectroscopic techniques, including 1H and 13C-NMR, FT-IR, mass, TGA, microanalysis and UV–Vis spectroscopy. The various characterization data suggested that Schiff base ligands coordinated through O atom of carbonyl group, N atom of azomethine and deprotonated phenolic O atoms with Ru metal ion in (2:1) molar ratio having the general formulas [Ru(p-cymene)L1]∙Cl∙0.5H2O and [RuCl(p-cymene)L2]∙Cl. Molecular structures of KB-1 and KB-2 complexes were found as pseudo-octahedral geometry. The complexes were screened as catalysts in the TH reactions. The results have revealed that KB-1 and KB-2 complexes showed efficient catalytic activity for the TH of ketones.
Graphical Abstract





Similar content being viewed by others
References
Aranda B, Valdebenito G, Parra-Melipán S, López V, Moya S, Vega A, Aguirre P (2022) Mol Catal 526:112374. https://doi.org/10.1016/j.mcat.2022.112374
Krishnaraj S, Muthukumar M, Viswanathamurthi P, Sivakumar S (2008) Transit Met Chem 33(5):643–648. https://doi.org/10.1007/s11243-008-9091-x
Chelopo MP, Pawar SA, Sokhela MK, Govender T, Kruger HG, Maguire GEM (2013) Eur J Med Chem 66:407–414. https://doi.org/10.1016/j.ejmech.2013.05.048
Alkabli J, Rizk M, Elshaarawy RFM, El-Sayed WN (2021) Int J Bio Macromol 184:454–462. https://doi.org/10.1016/j.ijbiomac.2021.06.105
Arish D, Sivasankaran NM (2010) J Mol Struct 983:112–121. https://doi.org/10.1016/j.molstruc.2010.08.040
Subarkhan MKM, Ren L, Xie B, Chen C, Wang Y, Wang H (2019) Eur J Med Chem 179:246–256. https://doi.org/10.1016/j.ejmech.2019.06.061
Maji M, Acharya S, Maji S, Purkait K, Gupta A, Mukherjee A (2020) Inorg Chem 59:10262–10274. https://doi.org/10.1021/acs.inorgchem.0c01433
Novakova O, Chen H, Vrana O, Rodger A, Sadler PJ, Brabec V (2003) Biochem 42:11544–11554. https://doi.org/10.1021/bi034933u
Savcı A, Buldurun K, Alkış ME, Alan Y, Turan N (2022) Med Oncol 39:172. https://doi.org/10.1007/s12032-022-01774-0
Bhaskar RS, Ladole CA, Salunkhe NG, Barabde JM, Aswar AS (2020) Arab J Chem 13(8):6559–6567. https://doi.org/10.1016/j.arabjc.2020.06.012
Acharya S, Moumita M, Ruturaj PK, Gupta A, Mukherjee A (2019) Inorg Chem 58(14):9213–9224. https://doi.org/10.1021/acs.inorgchem.9b00853
Çolak N, Karayel A, Buldurun K, Turan N (2021) J Struct Chem 62:37–46. https://doi.org/10.1134/S0022476621010054
Turan N, Buldurun K, Türkan F, Aras A, Çolak N, Murahari M, Bursal E, Mantarcı A (2022) Mol Divers 26:2459–2472. https://doi.org/10.1007/s11030-021-10344-x
Ereshanaik MC, Prabhakara HS, Bhojya Naik BR, Kirthan HM, Kumaraswamy R, Jain SK (2022) J India Chem Soc 99:100288. https://doi.org/10.1016/j.jics.2021.100288
Ghanghas P, Choudhary A, Kumar D, Poonia K (2021) Inorg Chem Commun 130:108710. https://doi.org/10.1016/j.inoche.2021.108710
Buldurun K, Özdemir M (2020) J Mol Struct 1202:127266. https://doi.org/10.1016/j.molstruc.2019.127266
Buldurun K, Turan N, Savci A, Alan Y, Çolak N (2023). Iran J Chem Chem Eng. https://doi.org/10.30492/IJCCE.2021.531629.4775S
Vijayapritha S, Viswanathamurthi P (2020) J Org Chem 929:121555. https://doi.org/10.1016/j.jorganchem.2020.121555
Yadav S, Vijayan P, Gupta R (2021) J Organomet Chem 954–955:122081. https://doi.org/10.1016/j.jorganchem.2021.122081
Thirumal M, Venkattappan A, Venkatachalam G (2020) J Organomet Chem 923:121408. https://doi.org/10.1016/j.jorganchem.2020.121408
Rüther T, Woodward CP, Jones TW, Coghlan CJ, Hebting Y, Cordiner RL, Dawson RE, Robinson DEJE, Wilson GJ (2016) J Organomet Chem 823:136–146. https://doi.org/10.1016/j.jorganchem.2016.08.030
Ramesh M, Venkatachalam G (2019) J Organomet Chem 880:47–55. https://doi.org/10.1016/j.jorganchem.2018.10.029
Buldurun K, Turan N, Savcı A, Çolak N (2019) J Saudi Chem Soc 23:205–214. https://doi.org/10.1016/j.jscs.2018.06.002
Buldurun K, Özdemir İ DUBITED 7:63–72 https://dergipark.org.tr/en/pub/dubited/issue/44006/473988 (2019)
Buldurun K, Turan N, Mahmoudi G, Bursal E (2022) J Mol Struct 1262:133075. https://doi.org/10.1016/j.molstruc.2022.133075
Turan N, Buldurun K (2018) Eur J Chem 9:22–29. https://doi.org/10.5155/eurjchem.9.1.22-29.1671
Buldurun K, Turan N, Çolak N, Özdemir İ (2019) DEU FMD 21:73–82. https://doi.org/10.21205/deufmd.2019216108
Turan N, Buldurun K, Çolak N, Özdemir İ (2019) Open Chem 17:571–580. https://doi.org/10.1515/chem-2019-0074
Sarıdağ T.: Master Thesis, Institute of Science and Technology, Muş Alparslan University, Muş/Turkey (2022)
Naskar R, Ghosh P, Manna CK, Murmu N, Mondal TK (2022) Inorganica Chim Acta 534:120802. https://doi.org/10.1016/j.ica.2022.120802
Pandiarajan D, Ramesh R (2013) J Organomet Chem 723:26–35. https://doi.org/10.1016/j.jorganchem.2012.10.003
Jayaseelan P, Akila E, Usha Rani M, Rajavel R (2016) J Saudi Chem Soc 20(6):625–634. https://doi.org/10.1016/j.jscs.2013.07.001
Nandhini R, Krishnamoorthy BS, Venkatachalam G (2019) J Organomet Chem 903:120984. https://doi.org/10.1016/j.jorganchem.2019.120984
Chen C, Ji J, Wang C-J, Jia A-Q, Zhang Q-F (2020) J Organomet Chem 910:121129. https://doi.org/10.1016/j.jorganchem.2020.121129
Bacchi A, Pelagatti P, Pelizzi C, Rogolino D (2009) J Organomet Chem 694:3200–3211. https://doi.org/10.1016/j.jorganchem.2009.05.010
Nagalakshmi V, Nandhini R, Brindha V, Krishnamoorthy BS, Balasubramani K (2020) J Organomet Chem 912:121175. https://doi.org/10.1016/j.jorganchem.2020.121175
Premkumar M, Vijayan P, Venkatachalam G (2019) J Organomet Chem 902:120964. https://doi.org/10.1016/j.jorganchem.2019.120964
Gichumbi JM, Friedrich HB, Omondi B (2016) J Mol Catal A Chem 416:29–38. https://doi.org/10.1016/j.molcata.2016.02.012
Al-Saif FA, Alibrahim KA, Alosaimi EH, Assirey E, El-Shahawi MS, Refat MS (2018) J Mol Liq 266:242–251. https://doi.org/10.1016/j.molliq.2018.06.077
de Araujo MP, de Figueiredo AT, Bogado AL, Von Poelhsitz G, Ellena J, Castellano EE, Claudio DL, Donnici L, Joao CV, Batista AA (2005) Organometallics 24(25):6159–6168. https://doi.org/10.1021/om050182b
Zacharopoulos N, Kolovou E, Peppas A, Koukoulakis K, Bakeas E, Schnakenburg G, Philippopoulos AI (2018) Polyhedron 154:27–38. https://doi.org/10.1016/j.poly.2018.07.030
Acknowledgements
This study was supported by Muş Alparslan University (Turkey) Research Fund (BAP-20-TBMY-4902-03). The authors thank the Institute of Science for their postgraduate studies and the Department of Chemistry of the Faculty of Arts and Sciences of Muş Alparslan University for the characterization of the compounds (FT-IR and UV-Vis Spectrophotometer analysis).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no competing financial interests or personal conflicts that could affect the work presented in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarıdağ, T., Buldurun, K. New Ruthenium-p-Cymene Complexes Containing o-Vanillin and 4-Benzoxybenzaldehyde Schiff Base Ligands; Synthesis, Characterization and Catalytic Activity in the Transfer Hydrogenation of Ketones. Catal Lett 154, 107–115 (2024). https://doi.org/10.1007/s10562-023-04286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04286-2