Abstract
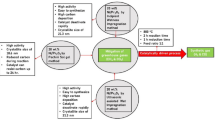
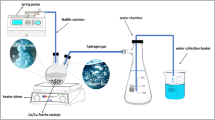
In this study, the production of two-stage metal-free catalysts from pistachio shells, which are abundant in Turkey and the world, is designed. The first stage of the study includes activated carbon(AC) production, and the second stage includes the production of heteroatom-doped catalysts as a result of hydrothermal heating of the obtained activated carbon with nitric acid. These metal-free catalysts obtained were used for the production of hydrogen (H2) from sodium borohydride (NaBH4) in methanol. In H2 production experiments, the NaBH4 concentration effect, temperature effect and catalyst amount effect and catalyst reusability parameters were investigated. HGR values obtained from the methanolysis using metal-free catalysts treated by the nitric acid/water ratio: 1:2, 1:3, 1:4, 1:5 are 8041, 8955, 10600 and 8333 ml min−1 g−1, respectively. The activation energy (Ea) of the production of H2 from NaBH4(NaBH4–H2–P) in methanol by the metal-free catalyst was 18.19 kJ mol−1. Fourier-transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), CHNS elemental analysis, scanning electron microscopy (SEM), and nitrogen adsorption analyses were performed for the characterization of the metal-free catalysts obtained. In addition, the mechanism of the obtained metal-free catalysts on the NaBH4–H2–P in methanol is discussed.
Graphical Abstract













Similar content being viewed by others
References
Barreto L, Makihira A, Riahi K (2003) The hydrogen economy in the 21st century: a sustainable development scenario. Int J Hydrogen Energy 28:267–284. https://doi.org/10.1016/S0360-3199(02)00074-5
Ji J, Deng K, Li J et al (2021) In situ transformation of 3D Co3O4 nanoparticles to 2D nanosheets with rich surface oxygen vacancies to boost hydrogen generation from NaBH4. Chem Eng J. https://doi.org/10.1016/J.CEJ.2021.130350
Selvitepe N, Balbay A, Saka C (2019) Optimisation of sepiolite clay with phosphoric acid treatment as support material for CoB catalyst and application to produce hydrogen from the NaBH 4 hydrolysis. Int J Hydrog Energ. https://doi.org/10.1016/j.ijhydene.2019.04.254
Saka C, Salih Eygi M, Balbay A (2020) CoB doped acid modified zeolite catalyst for enhanced hydrogen release from sodium borohydride hydrolysis. Int J Hydrog Energ. https://doi.org/10.1016/j.ijhydene.2020.03.238
Colak TO, Tuc Altaf C, Minkina VG et al (2022) Efficient hydrogen generation with Co3O4@TiO2-g-C3N4 composite catalyst via catalytic NaBH4 hydrolysis. Catal Lett 152:2779–2788. https://doi.org/10.1007/S10562-021-03848-6/FIGURES/6
Saka C, Balbay A (2020) Influence of process parameters on enhanced hydrogen evolution from alcoholysis of sodium borohydride with a boric acid catalyst. Int J Hydrog Energ 45:16193–16200. https://doi.org/10.1016/j.ijhydene.2020.04.094
Li Q, Li Y, Chen J et al (2022) Synergistically photo-thermo-catalytic effect of metal-oxide semiconductors with d 10 electronic configuration for hydrogen generation in NaBH4 hydrolyzation. Catal Lett 152:2401–2411. https://doi.org/10.1007/S10562-021-03825-Z/FIGURES/10
Zhou J, Yan J, Meng X et al (2022) Co0.45W0.55 nanocomposite from ZIF-67: an efficient and heterogeneous catalyst for H2 generation upon NaBH4 hydrolysis. Catal Lett 152:610–618. https://doi.org/10.1007/S10562-021-03661-1/FIGURES/8
Crisafulli C, Scirè S, Zito R, Bongiorno C (2012) Role of the support and the Ru precursor on the performance of ru/carbon catalysts towards H2 production through NaBH4 hydrolysis. Catal Lett 142:882–888. https://doi.org/10.1007/s10562-012-0844-y
Xu D, Zhang Y, Guo Q (2022) Research progress on catalysts for hydrogen generation through sodium borohydride alcoholysis. Int J Hydrog Energ 47:5929–5946. https://doi.org/10.1016/J.IJHYDENE.2021.11.232
Khan SB (2020) Metal nanoparticles containing chitosan wrapped cellulose nanocomposites for catalytic hydrogen production and reduction of environmental pollutants. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2020.116286
Sahiner N, Demirci S (2017) Natural microgranular cellulose as alternative catalyst to metal nanoparticles for H2production from NaBH4methanolysis. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2016.09.028
Meşe E, Kantürk Figen A, Coşkuner Filiz B, Pişkin S (2018) Cobalt-boron loaded thermal activated Turkish sepiolite composites (Co-B@tSe) as a catalyst for hydrogen delivery. Appl Clay Sci. https://doi.org/10.1016/j.clay.2017.12.008
Kassem AA, Abdelhamid HN, Fouad DM, Ibrahim SA (2019) Metal-organic frameworks (MOFs) and MOFs-derived CuO@C for hydrogen generation from sodium borohydride. Int J Hydrog Energ. https://doi.org/10.1016/j.ijhydene.2019.10.047
Dai P, Yao Y, Hu E et al (2021) Self-assembled ZIF-67@graphene oxide as a cobalt-based catalyst precursor with enhanced catalytic activity toward methanolysis of sodium borohydride. Appl Surf Sci 546:149128. https://doi.org/10.1016/j.apsusc.2021.149128
Paraknowitsch JP, Thomas A (2013) Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energ Environ Sci 6:2839–2855. https://doi.org/10.1039/c3ee41444b
Xie X, Shi J, Pu Y et al (2020) Cellulose derived nitrogen and phosphorus co-doped carbon-based catalysts for catalytic reduction of p-nitrophenol. J Colloid Interface Sci 571:100–108. https://doi.org/10.1016/J.JCIS.2020.03.035
Queiroz LS, de Souza LKC, Thomaz KTC et al (2020) Activated carbon obtained from amazonian biomass tailings (acai seed): modification, characterization, and use for removal of metal ions from water. J Environ Manage 270:110868. https://doi.org/10.1016/J.JENVMAN.2020.110868
Faulconer EK, Mazyck DW (2017) Influence of activated carbon surface oxygen functionality on elemental mercury adsorption from aqueous solution. J Environ Chem Eng 5:2879–2885. https://doi.org/10.1016/J.JECE.2017.05.036
Ukanwa KS, Patchigolla K, Sakrabani R et al (2019) A review of chemicals to produce activated carbon from agricultural waste biomass. Sustain 11:6204. https://doi.org/10.3390/SU11226204
Qiu Y, Ali S, Lan G et al (2019) Defect-rich activated carbons as active and stable metal-free catalyst for acetylene hydrochlorination. Carbon N Y 146:406–412. https://doi.org/10.1016/J.CARBON.2019.01.102
Xu N, Zhu M, Zhang J et al (2015) Nitrogen functional groups on an activated carbon surface to effect the ruthenium catalysts in acetylene hydrochlorination. RSC Adv 5:86172–86178. https://doi.org/10.1039/C5RA18851B
Yuan C, Liu X, Jia M et al (2015) Facile preparation of N- and O-doped hollow carbon spheres derived from poly(o-phenylenediamine) for supercapacitors. J Mater Chem A 3:3409–3415. https://doi.org/10.1039/C4TA06411A
Han Y, Zhang Z, Guo L (2021) Electrocatalytic CO2 reduction activity over transition metal anchored on nitrogen-doped carbon: a density functional theory investigation. Catal Lett 151:2547–2559. https://doi.org/10.1007/S10562-020-03498-0/FIGURES/6
Mao D, Jia M, Qiu J et al (2020) N-doped porous carbon supported au nanoparticles for benzyl alcohol oxidation. Catal Lett 150:74–81. https://doi.org/10.1007/S10562-019-02949-7/FIGURES/5
Qiu Z, Ma S, He X et al (2022) Nitrogen-doped porous two-dimensional carbon nanosheets derived from ZIF-8 as multifunctional supports of Ru nanoparticles for hydrogenation of benzoic acid. Catal Lett 1:1–10. https://doi.org/10.1007/S10562-022-03982-9/TABLES/6
Long D, Wang L, Cai H et al (2020) Sulfur doped carbon-rich g-c3n4 for enhanced photocatalytic h2 evolution: morphology and crystallinity effect. Catal Lett 150:2487–2496. https://doi.org/10.1007/S10562-020-03156-5/TABLES/2
El-Hendawy ANA (2003) Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon NY 41:713–722. https://doi.org/10.1016/S0008-6223(03)00029-0
Figueiredo J, Pereira MF, Freitas MM, Órfão JJ (1999) Modification of the surface chemistry of activated carbons. Carbon NY 37:1379–1389. https://doi.org/10.1016/S0008-6223(98)00333-9
Zhang S, Sheng K, Yan W et al (2021) Bamboo derived hydrochar microspheres fabricated by acid-assisted hydrothermal carbonization. Chemosphere 263:128093. https://doi.org/10.1016/J.CHEMOSPHERE.2020.128093
Li X, Li MF, Bian J et al (2015) Hydrothermal carbonization of bamboo in an oxalic acid solution: Effects of acid concentration and retention time on the characteristics of products. RSC Adv 5:77147–77153. https://doi.org/10.1039/C5RA15063A
(2019) http://www.fao.org/faostat/en/#data/QCL. http://www.fao.org/faostat/en/#data/QCL.
Kasiri N, Fathi M (2018) Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int J Biol Macromol 106:1023–1031. https://doi.org/10.1016/J.IJBIOMAC.2017.08.112
Apaydin-Varol E, Pütün E, Pütün AE (2007) Slow pyrolysis of pistachio shell. Fuel 86:1892–1899. https://doi.org/10.1016/J.FUEL.2006.11.041
Açikalin K, Karaca F, Bolat E (2012) Pyrolysis of pistachio shell: Effects of pyrolysis conditions and analysis of products. Fuel 95:169–177. https://doi.org/10.1016/J.FUEL.2011.09.037
Hu C, Liu Y, Yang Y et al (2012) One-step preparation of nitrogen-doped graphene quantum dots from oxidized debris of graphene oxide. J Mater Chem B 1:39–42. https://doi.org/10.1039/C2TB00189F
NOPR: Surface modification of granular activated carbon by nitric acid for the enhancement of copper adsorption. http://nopr.niscair.res.in/handle/123456789/20040. Accessed 11 Oct 2021
Shen Y, Yu Y, Zhang Y et al (2021) Role of redox-active biochar with distinctive electrochemical properties to promote methane production in anaerobic digestion of waste activated sludge. J Clean Prod 278:123212. https://doi.org/10.1016/J.JCLEPRO.2020.123212
Shen J, Huang G, An C et al (2017) Immobilization of tetrabromobisphenol a by pinecone-derived biochars at solid-liquid interface: Synchrotron-assisted analysis and role of inorganic fertilizer ions. Chem Eng J 321:346–357. https://doi.org/10.1016/J.CEJ.2017.03.138
Tian Z, Li J, Zhu G et al (2015) Facile synthesis of highly conductive sulfur-doped reduced graphene oxide sheets. Phys Chem Chem Phys 18:1125–1130. https://doi.org/10.1039/C5CP05475C
Song X, Liu H, Cheng L, Qu Y (2010) Surface modification of coconut-based activated carbon by liquid-phase oxidation and its effects on lead ion adsorption. Desalination 255:78–83. https://doi.org/10.1016/J.DESAL.2010.01.011
Li ZQ, Lu CJ, Xia ZP et al (2007) X-ray diffraction patterns of graphite and turbostratic carbon. Carbon NY 45:1686–1695. https://doi.org/10.1016/J.CARBON.2007.03.038
Muniandy L, Adam F, Mohamed AR, Ng EP (2014) The synthesis and characterization of high purity mixed microporous/mesoporous activated carbon from rice husk using chemical activation with NaOH and KOH. Microporous Mesoporous Mater 197:316–323. https://doi.org/10.1016/J.MICROMESO.2014.06.020
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2003) Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon N Y 41:267–275. https://doi.org/10.1016/S0008-6223(02)00279-8
Oginni O, Singh K, Oporto G et al (2019) Influence of one-step and two-step KOH activation on activated carbon characteristics. Bioresour Technol Rep 7:100266. https://doi.org/10.1016/J.BITEB.2019.100266
Arango DI, Zapata-Benabithe Z, Arenas EC, Perez-Osorno JC (2018) Influence of surface modification with nitric acid on electrochemical performance of agroindustrial waste-based activated carbon. J Mater Sci Mater Electron 29:15557–15569. https://doi.org/10.1007/S10854-018-9132-Y/FIGURES/9
Gao B, Wang Y, Huang L, Liu S (2021) Study on the performance of HNO3-modified biochar for enhanced medium temperature anaerobic digestion of food waste. Waste Manag 135:338–346. https://doi.org/10.1016/J.WASMAN.2021.09.020
Zhou H, Brown RC, Wen Z (2020) Biochar as an Additive in Anaerobic Digestion of Municipal Sludge: Biochar Properties and Their Effects on the Digestion Performance. ACS Sustain Chem Eng 8:6391–6401. https://doi.org/10.1021/ACSSUSCHEMENG.0C00571
El-Hendawy ANA (2003) Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon NY 41:713–722. https://doi.org/10.1016/S0008-6223(03)00029-0
Vanyorek L, Muránszky G, Fiser B et al (2019) Adsorption capacity of oxidized nitrogen-doped bamboo-like carbon nanotubes. J Dispers Sci Technol 41:1879–1884. https://doi.org/10.1080/01932691.2019.1637757
Kim J-H, Hwang SY, Park JE et al (2019) (2019) Impact of the oxygen functional group of nitric acid-treated activated carbon on KOH activation reaction. Carbon Lett 293(29):281–287. https://doi.org/10.1007/S42823-019-00024-0
Saka C (2022) Facile fabrication of P-doped g-C3N4 particles with nitrogen vacancies for efficient dehydrogenation of sodium borohydride methanolysis. Fuel 313:122688. https://doi.org/10.1016/J.FUEL.2021.122688
Saka C (2021) Metal-free catalysts with phosphorus and oxygen doped on carbon-based on Chlorella Vulgaris microalgae for hydrogen generation via sodium borohydride methanolysis reaction. Int J Hydrog Energ. https://doi.org/10.1016/j.ijhydene.2020.09.220
Saka C (2021) Highly active and durable hydrogen release in NaBH4 methanolysis reaction with sulphur and phosphorus-doped metal-free microalgal carbon nanoparticles. Appl Catal B Environ 292:120165. https://doi.org/10.1016/j.apcatb.2021.120165
Saka C, Balbay A (2021) Oxygen and nitrogen-functionalized porous carbon particles derived from hazelnut shells for the efficient catalytic hydrogen production reaction. Biomass Bioenerg 149:106072. https://doi.org/10.1016/j.biombioe.2021.106072
Demirci S, Suner SS, Yildiz M, Sahiner N (2022) Polymeric ionic liquid forms of PEI microgels as catalysts for hydrogen production via sodium borohydride methanolysis. J Mol Liq 360:119562. https://doi.org/10.1016/J.MOLLIQ.2022.119562
Saka C (2022) Performance of g-C3N4 nanoparticles by EDTA modification and protonation for hydrogen release from sodium borohydride methanolysis. Int J Hydrog Energ 47:13654–13663. https://doi.org/10.1016/J.IJHYDENE.2022.02.121
Bakhsh EM, Khan MSJ, Akhtar K et al (2022) Chitosan hydrogel wrapped bimetallic nanoparticles based efficient catalysts for the catalytic removal of organic pollutants and hydrogen production. Appl Organomet Chem 36:e6741. https://doi.org/10.1002/AOC.6741
Saka C (2022) Surface modification with oxygen doping of g-C3N4 nanoparticles by carbon vacancy for efficient dehydrogenation of sodium borohydride in methanol. Fuel 310:122444. https://doi.org/10.1016/J.FUEL.2021.122444
Saka C, Balbay A (2022) Metal-free catalyst fabrication by incorporating oxygen groups on the surface of the carbonaceous sample and efficient hydrogen production from NaBH4 methanolysis. Int J Hydrog Energ 47:7242–7251. https://doi.org/10.1016/J.IJHYDENE.2021.12.070
Samatya Ölmez S, Balbay A, Saka C (2022) Phosphorus doped carbon nanodots particles based on pomegranate peels for highly active dehydrogenation of sodium borohydride in methanol. Int J Hydrog Energ. https://doi.org/10.1016/J.IJHYDENE.2022.07.091
Ozturk OF, Demirci S, Sengel SB, Sahiner N (2018) Highly regenerable ionic liquid microgels as inherently metal-free green catalyst for H2 generation. Polym Adv Technol 29:1426–1434. https://doi.org/10.1002/PAT.4254
Demirci S, Zekoski T, Sahiner N (2019) The preparation and use of p(2-acrylamido-2-methyl-1-propanesulfonic acid)-tris(dioxa-3,6-heptyl)amine (p(AMPS)-TDA-1) ionic liquid microgel in hydrogen production. Polym Bull 76:1717–1735. https://doi.org/10.1007/S00289-018-2465-0
Saka C, Kaya M, Bekiroğullari M (2020) Chlorella vulgaris microalgae strain modified with zinc chloride as a new support material for hydrogen production from NaBH4 methanolysis using CuB, NiB, and FeB metal catalysts. Int J Hydrog Energ 45:1959–1968. https://doi.org/10.1016/j.ijhydene.2019.11.106
Saka C (2021) Sulphur and nitrogen-doped metal-free microalgal carbon catalysts for very active dehydrogenation of sodium borohydride in methanol. Int J Hydrog Energ 46:18326–18337. https://doi.org/10.1016/j.ijhydene.2021.03.001
Saka C (2021) Oxygen and nitrogen-doped metal-free microalgae carbon nanoparticles for efficient hydrogen production from sodium borohydride in methanol. Int J Hydrog Energ 46:26298–26307. https://doi.org/10.1016/j.ijhydene.2021.05.111
Saka C (2021) Very efficient dehydrogenation of methanolysis reaction with nitrogen doped Chlorella Vulgaris microalgae carbon as metal-free catalysts. Int J Hydrog Energ 46:20961–20971. https://doi.org/10.1016/J.IJHYDENE.2021.03.220
Sahiner N (2017) Modified multi-wall carbon nanotubes as metal free catalyst for application in H2 production from methanolysis of NaBH4. J Power Sources 366:178–184. https://doi.org/10.1016/j.jpowsour.2017.09.041
Abebe MW, Baye AF, Kim H (2022) Poly (acrylic acid)/polysaccharides IPN derived metal free catalyst for rapid hydrogen generation via NaBH4 methanolysis. Int J Hydrog Energ. https://doi.org/10.1016/J.IJHYDENE.2022.07.106
Saka C (2022) Phosphorus decorated g-C3N4-TiO2 particles as efficient metal-free catalysts for hydrogen release by NaBH4 methanolysis. Fuel 322:124196. https://doi.org/10.1016/J.FUEL.2022.124196
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of CS. The author has approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saka, C. Nitrogen and Oxygen Heteroatom Doping with Hydrothermal Nitric Acid Treatment on the Catalytic Performance of Metal-Free Carbon Particles: Hydrogen Release from Sodium Borohydride in Methanol. Catal Lett 153, 3734–3749 (2023). https://doi.org/10.1007/s10562-023-04277-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04277-3