Abstract
To prepare activated carbon with a high specific surface area, oxygen functional groups (OFGs) that can serve as useful electron donors during KOH activation were treated with nitric acid and incorporated into activated carbon. OFGs are incorporated differently according to the surface characteristics of starting materials. Up to 22.46% OFGs are incorporated into wood-based activated carbons (WACs), the C=O, COOH contents was 1.90, 17.05%, respectively. Whereas up to 12.82% OFGs are incorporated into coconut shell-based activated carbons, the C=O, COOH contents was 4.12, 6.15%, respectively. The OFGs used for increasing the specific surface area are the carbonyl group, and as the content of the functional group increases, the carbonyl group spreads to the carboxyl group. The specific surface area of activated carbons increased by 10–68% with an increase in the carbonyl group up to 6% (maximum point of carbonyl group). On the other hand, the specific surface area for WACs increased when the carboxyl group was 10% or below, but decreased by 6–15% when it increased to 10% or excess.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Activated carbons are carbon solids obtained by treating wood, peat, petroleum pitch, or nut shells with a structure consisting mainly of carbons through high-temperature activation reaction. The activated carbons obtained after the activation reaction form porous materials. Therefore, activated carbons have been used for water purification and as adhesive materials that can attach and remove volatile organic compounds. In recent times, activated carbons have been used as an energy-storing medium with storage restrictions, and there is a rapid increase in their use in the air purifier and automobile filter field. Therefore, there is a demand for the development of high specific-surface area activated carbons in these fields [1].
The specific surface area of activated carbons shows differences in degree according to the property of the starting materials. The specific surface area of coconut shells is between 900 and 1100 m2/g, whereas the specific surface area of wood-based activated carbons (WAC) is relatively higher at 1500–1600 m2/g. The volatile matter content in WAC is approximately 7% higher than that in coconut shell-based activated carbons (CAC). The specific surface area of WAC activated using KOH is approximately 3000 m2/g and that of CAC is approximately 2000 m2/g. Therefore, the specific surface area of WAC has been reported to be approximately 50% higher than that of CAC [2, 3].
The volatile matter of activated carbons is known to be affected by the content of the oxygen functional group (OFG) on the carbon surface. For example, a previous study incorporated OFGs into activated carbons through nitric acid treatment. Ternero-Hidalgo et al. added olive-stone-based activated carbons to a nitric acid solution and stirred it at 80 °C for surface treatment. Various functional groups are formed according to the mol of nitric acid, which reacts with activated carbons. If the mol of nitric acid is low, nitro groups and ketones are formed (–CH2 + 2HNO3 → C=O + 2HNO2 + H2O, –CH + HNO3 → –C–NO2 + H2O). As the mol of nitric acid increases, carboxyl groups (–2CH2 + 5HNO3 → 2COOH + 5HNO2 + H2O) are formed [4,5,6]. The activation reactions resulting from alkali metals on the surface of carbon precursors are accompanied by oxidation–reduction reactions that form pores, and such oxidation–reduction reactions are affected by the surface characteristics of activated carbon (e.g., electron donors). Falco et al. reported that OFGs on the surface of precursors increased as heat treatment of carbon precursors advanced from 180 to 240 °C, and that pore formation increased owing to the increase in OFGs. They emphasized the usefulness of carbonyl groups among all OFGs. Subsequently, when the heat treatment temperature was increased to 280 °C or higher, the structural stability of precursors improved and the chemical surface reactions decreased, which reduced pore formation [7].
Shafeeyan et al. proposed different types of OFGs incorporated into activated carbons and demonstrated the types of volatile gases that may be generated and the temperature at which volatile gas is generated according to the form of functional groups through temperature-programmed desorption. Here, if the OFGs incorporated into activated carbons are carbonyl groups, carbon monoxide will be formed, but if they are carboxy groups or lactone groups, carbon dioxide will be formed instead. Moreover, radical and open pores were observed to form as OFGs at the carbon surface became volatilized through heat treatment. At this point, as CO was formed for the carbonyl groups and ketones, open pores were formed more easily, but as CO2 was formed for quinones and carboxyl groups, the carbon bond was removed and open pore formation was not easy [8, 9].
In this study, activated carbons (wood and coconut shell) were acid-treated using nitric acid solutions at various concentrations and OFGs were incorporated, and a KOH activation reaction was induced in order to prepare activated carbons with a high specific surface area. To verify the reactivity with nitric acid according to the basic properties of activated carbons, a proximate analysis and elementary analysis were performed on WAC and CAC, and the OFGs formed on the activated carbons through nitric acid treatment were verified using X-ray photoelectron spectroscopy (XPS) analysis. Consequently, the specific surface area and pore size distribution of the final activated carbons were verified to examine the effect of the form and content of OFGs in activated carbons on the KOH activation reaction.
2 Materials and methods
2.1 Materials
To prepare activated carbons with a high specific surface area, WAC (Ja Yeon Science Ind, Co., JIG-SC-2040 BT, 20–40 mesh, 1400 m2/g, 0.2 g/cc) and CAC (Ja Yeon Science Ind, Co., JIG-SC-2040, 20–40 mesh, 1000 m2/g, 0.5 g/cc) were used as the carbon precursors. The activated carbon manufacturing process used the each of coconut shell-based charcoal and wood as raw materials and proceeded with carbonization and activation process. The carbonization process was the heating of the raw materials at 500 °C, the activation process was the heating of the fixed carbons at temperature between 950 and 980 °C in an inert atmosphere using steam. To incorporate oxygen functional groups into activated carbons, nitric acid (Oriental chemical, 60%) was used as the surface treatment agent. To activate these activated carbons, potassium hydroxide (KOH, Samchun chemical, 95%) was used as the activation agent.
2.2 Preparations of activated carbons by HNO3 treatment and KOH activation
Commercial activated carbons based on wood and coconut shells were selected as starting materials. All the activated carbons were ground and only those with a particle size in the range 20–40 mesh were used in the experiment. The OFGs were incorporated on the surface of activated carbons using a nitric acid (HNO3) solution. The WAC or CAC (3 g) and nitric acid solution (5, 10, 20%, 60 mL) were placed inside a 100 mL Erlenmeyer flask and stirred and heated at 80 °C for 12 h. After the surface-treated activated carbons were washed multiple times with distilled water until they became neutral (pH 7.0), they were dehydrated in an oven heated to 105 °C for at least 12 h. The samples obtained according to the nitric acid treatment concentration conditions were named WAC-N5, WAC-N10, WAC-N20, CAC-N5, CAC-N10, and CAC-N20. The surface-treated activated carbons underwent an activation reaction by mixing KOH:activated carbon (KOH/AC-Ns = 1, 2, 3) to solid:solid. The activation reaction proceeded for 1 h at 750 °C and 3 h at 850 °C at a heating rate of 10 °C/min considering the material properties of KOH (K-vaporized at 761 °C). The reactants that finished the activation reaction were washed with a 1 M sulfuric acid solution and distilled water until they were neutralized (pH 7.0) and dehydrated at 105 °C (in Fig. 1). This entire process was repeated three times and the samples activated after acid treatment were named WAC-N5-K, WAC-N10-K, WAC-N20-K, CAC-N5-K, CAC-N10-K, and CAC-N20-K.
2.3 Analysis of activated carbons treated with HNO3 and KOH activation
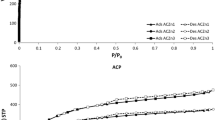
The proximate analysis (flammable matter, fixed carbon, and ash content) on WAC and CAC treated with nitric acid was performed based on the Weende method [1]. The elementary analysis was performed using the element content of C, H, N, S, and O using an elemental analyzer (Thermo scientific, FlashEA 1112). XPS (Thermo scientific, VG Multilab 2000) was used for the qualitative/quantitative analysis of the surface functional groups of activated carbons. An X-ray diffractometer (XRD, Rigaku, SmartLab) was used for the analysis of the crystal structure and Bragg’s and Sherrer’s laws were used for the analysis of each crystal’s size and layer spacing, respectively. A specific surface area analyzer (ASAP2010, Micromeritics Co.) was used for measuring the specific surface area. The absorption volume was obtained according to changes in pressure (P/P0) after pre-treatment from a vacuum condition of 350 °C (< 2.7 × 10−3 kPa), and the nitrogen absorption single-layer volume was calculated using the Brunauer–Emmett–Teller method.
3 Results and consideration
3.1 Proximate analysis and elementary analysis of nitric acid-treated activated carbons
The results of the proximate analysis and elementary analysis of activated carbons used in this study are shown in Table 1. The elementary composition showed differences depending on the starting materials used for preparing activated carbons; the fixed carbon and carbon content of CAC were higher than those of WAC. Volatile matter was 10.48 ± 0.14% on average for WAC, which is approximately 3 times higher than the 2.96 ± 0.08% average volatile matter for CAC, and there was a drastic increase owing to surface treatment using nitric acid. In the results of the elementary analysis, there was an apparent increase in oxygen, which showed that the incorporation of surface functional groups (OFGs) was seamless through nitric acid treatment. There was a distinct difference in the increased oxygen content according to the type of activated carbons. Therefore, the volatile matter (OFGs) that increased through surface treatment contributed to the formation of defect areas during the activation process; hence, a high specific surface area is expected to form compared with the initial conditions through an activation reaction.
3.2 Functional group structure of nitric acid-treated activated carbons
In the XPS analysis results, there was a distinct increase in OFGs (O1s) according to the degree of nitric acid treatment in the two types of activated carbons as in the results of the elementary analysis. The “OTotal/CTotal ratio” from the measured C1s and O1s could be used as an indicator of the degree of surface oxidation, and the OTotal/CTotal ratio continuously increased according to the degree of surface treatment regardless of the type of activated carbon (Table 2). The ratio for wood was approximately 1.4 times higher than that for coconut shells. This ratio is believed to be related to the physical characteristics of the production materials of activated carbons (relative critical strength according to carbonization). The introduction of hydrophilic functional groups into the activated carbons is known to be influenced by the surface characteristics (polarity) of the raw materials and the contents of the heterogeneous compositions (H, N, S, O…) [7, 10]. Each activated carbon was prepared by the same process; however, it was confirmed that WAC (Ash: 3.39%) having a high ash contents (SiO2) had higher contents of hydrophilic functional groups and heterogeneous compositions than that of CAC (Ash: 2.05%). Therefore, WAC seemed to be easier to introduce the hydrophilic functional groups in the aqueous solution. The oxidation of carbon solids verified through changes in the “OTotal/CTotal ratio” partially affected the structure of amorphous carbon solids. Upon illustrating the relationship between C=C(sp2) and C–C(sp3) by deconvolutionalizing the C1s superimposed peak according to the degree of surface treatment in this study, an inverse correlation is observed (R2 = 0.728) (Fig. 2). This effectively explains that there are structural changes in carbon solids through surface oxidation. The content of functional groups on the surface of carbon solids (C–O, C=O, COOH) also showed distinct changes. The C=C(sp2), C–C(sp3), C–O, C=O, and COOH content in WAC was 62.99, 16.05, 8.00, 6.85, and 6.12%, respectively. Subsequently, the content of the carbonyl group decreased from 6.85% (starting materials) to 1.90% (20% nitric acid) as nitric acid treatment progressed for WAC. The carboxyl group content showed a distinct increase from 6.12% (starting materials) to 17.05% (20% nitric acid). While there are differences in the content of incorporated OFGs, a similar trend was observed for CAC. However, unlike WAC, there was a relative resistance against oxidation reactions through the incorporation of surface functional groups resulting from nitric acid processing for CAC due to low amounts of OFGs as electron donors incorporated in CAC compared to WAC [7]. For CAC surface treated with the same 20% nitric acid concentration, the –COOH functional group content was approximately 3 times less than that of WAC.
3.3 Crystal structures and specific surface areas of activated carbons treated with HNO3
Activated carbons with a turbostatic structure are generally amorphous, and XRD analysis was performed to observe changes in the relative crystal structure of activated carbons through surface treatment (Fig. 3). The amorphous peak of activated carbons was observed from the crystal faces of (002) and (101) at 22° and 43°, respectively. The layer spacing of activated carbons from peak (002) was shown through the Bragg Formula (1) and the crystal size was shown through the Scherrer Formula (2) (Table 3).
where λ is the emission wavelength and has a value of 1.5406, and θ is the angle of reflection.
where β002 refers to the half width of peak (002).
Consequently, WAC showed a layer spacing of 0.3973 nm and a crystal size of 0.941 nm, whereas CAC showed a layer spacing of 0.3907 nm and crystal size of 0.974 nm. Based on the above results, there were no major differences in layer spacing or crystal size between the two activated carbons with a turbostatic structure. Subsequently, the crystal size of WAC-N10 and CAC-N10 was calculated as 0.793 nm and 0.861 nm, respectively, by performing nitric acid treatment on activated carbons. These values were compared with the crystal size of the starting materials, which showed that there was a decrease of approximately 0.148 nm and 0.113 nm, respectively. Activated carbons cause the structure collapses as an oxidation reaction was conducted by nitric acid treatment, which is believed to cause a decrease in crystal size [11]. And then, the interlayer spacings (d002) of the WAC-N10-K and CAC-N10-K were largely increased by changing the C=C(sp2) bond to C–C(sp3) bond through activation reaction, it was also observed that the crystal sizes (Lc) were increased. The increased in interlayer spacings and crystal sizes of the WAC-N10-K was amplified through the large oxidation reaction from nitric acid treatment for WAC-N10.
It was also confirmed that the specific surface areas of the nitric acid-treated activated carbons were decreased as compared with that of the untreated activated carbon (Table 4). This phenomenon appeared to be due to blocking the pores of the newly formed oxygen functional groups of the activated carbons or eroding the carbon skeletal structures [12]. In addition, the specific surface areas decreased significantly as the concentration of nitric acid treatment increased, WACs with the introduction of the high functional groups showed a significant decrease of the specific surface area compared with CACs.
3.4 Specific surface area of activated carbons treated with KOH
Micropores are known to form during the KOH activation process as metal potassium becomes reduced. Therefore, the OFGs incorporated through surface treatment can serve as useful electron donors during KOH activation. In other words, they are expected to contribute even more in the production of activated carbons with a high specific surface area. Table 5 shows the results of activation under KOH/AC-Ns = 3(w/w) conditions according to the degree of OFG incorporation. While there are differences in degree depending on the starting material, the specific surface area increased by approximately 2.05 times (WAC-K) and 1.68 times (CAC-K), respectively, compared with the starting materials (WAC and CAC) through only chemical activation without surface treatment. After activating the above surface-treated samples, the specific surface area and pore distribution of activated carbons showed different results depending on the degree of OFG incorporation in carbon precursors [12]. Although the specific surface area increased for KOH activation samples linked to surface treatment, there was a slight difference according to the target activated carbon. For WAC, the specific surface area of all surface-treated samples was lower than that of WAC-K, and the specific surface area increased up to 10% treatment concentration before falling at a concentration of 20%. For CAC, the increase in specific surface area according to the surface treatment concentration was similar to that for WAC, but the specific surface area was higher than that of CAC-K. This difference according to the target activated carbon seems to be related to the physical characteristics of the starting materials for producing activated carbons. The pore distribution pattern of WAC was developed for both micropores and mesopores after activation at existing mesopores, but the micropore and mesopore portions both decreased in surface-treated samples with high carboxylation reactivity (Fig. 4). The OFGs incorporated in the surface of carbon solids are known to contribute significantly to pore formation during the activation process, and carboxyl functional groups, which are already oxidized, cause the structure of the carbon solid to collapse during the activation process, which decreases the pore areas. The pore distribution of CAC was developed for both micropores and mesopores after activation at existing micropores, and the micropores in the surface-treated samples with low OFG incorporation expanded to show a distribution that was developed toward mesopores. For CAC, there was no major spread of carbonyl groups incorporated through surface treatment to carboxyl groups, which shows that pore development is easy compared with that in WAC as carbonyl groups are typically incorporated.
As the type of OFGs significantly impacts the development of the specific surface area of activated carbons, the KOH activation agent content (KOH/AC-Ns = 1, 2, 3 w/w) was used in various ways in surface-treated activated carbons to induce activation reactions in order to verify the specific surface area correlation of activated carbons according to the type of functional group. Table 5 shows the specific surface area. Under conditions with decreased activation agent content (KOH/AC-Ns = 1, 2 w/w), the development of specific surface area was greatest 0.18–35.58% for WAC-N5-K compared with WAC-K, into which the most carbonyl groups (5.69%) were incorporated (Table 2). Additionally, the development of specific surface area was greatest 0.34–24.73% for CAC-N5-K compared with CAC-K at the most carbonyl groups (4.75%) incorporated. While the specific surface area increased at the carboxyl group content of approximately 10% or below for WAC-N5-K, it began to decrease when the content rose to 10% or excess. However, under conditions with excessively increased activation agent content (KOH/AC-Ns = 3 w/w), specific surface area development through surface treatment did not show much. As explained in the above results, this phenomenon can be explained by the fact that carbonyl groups are useful in forming open pores out of OFGs, but if the content increases and spreads to carboxyl groups, pore formation becomes limited. Therefore, it is extremely important to control the type and content of functional groups for increasing the specific surface area of activated carbons under conditions with decreased activation agent content (KOH/AC-Ns = 1, 2 w/w).
4 Conclusion
To prepare activated carbon with a high specific surface area, OFGs that can serve as useful electron donors during KOH activation were treated with nitric acid and incorporated into activated carbon. When the treatment concentration increased, OFGs were incorporated differently according to the surface characteristics of the target activated carbons. The OTotal/CTotal ratio for wood was approximately 1.4 times higher than that for coconut shells. For CAC surface treated with the same 20% nitric acid concentration compared to WAC, the –COOH functional group content was approximately 3 times less than that of WAC. The OFGs used for increasing the specific surface area were the carbonyl group, and as the content of the functional group increased, the carbonyl group spread to the carboxyl group. Under conditions with decreased activation agent content (KOH/AC-Ns = 1, 2 w/w), the development of specific surface area was greatest 0.18–35.58% for WAC-N5-K compared with WAC-K, into which the most carbonyl groups (5.69%) were incorporated. Also, the development of specific surface area was greatest 0.34–24.73% for CAC-N5-K compared with CAC-K at the most carbonyl groups (4.75%) incorporated. While the specific surface area increased at the carboxyl group content of approximately 10% or below for WAC-N5-K, it began to decrease when the content rose to 10% or excess. The above results showed that the specific surface area can be increased by controlling the OFGs type and content of activated carbons.
References
Yang K, Peng J, Srinivasakannan C, Zhang L, Xia H, Duan X (2010) Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour Technol 101:6163. https://doi.org/10.1016/j.biortech.2010.03.001
Lee G, Jung HS, Hong B, Kim S, Choi S (2017) Optimization of washing process for the recycling of potassium in the manufacturing of activated carbon. J Korea Org Resour Recycl Assoc 25(3):63. https://doi.org/10.17137/korrae.2017.25.3.63
Chen J, Zhang L, Yang G, Wang Q, Li R, Lucia LA (2017) Preparation and characterization of activated carbon from hydrochar by phosphoric acid activation and its adsorption performance in prehydrolysis liquor. BioResources 12(3):5928. https://doi.org/10.15376/biores.12.3.5928-5941
Figueiredo JL, Pereira MFR, Freitas MMA, Orfao JJM (1999) Modification of the surface chemistry of activated carbons. Carbon 37:1379. https://doi.org/10.1016/S0008-6223(98)00333-9
Zhang X, Gao B, Creamer AE, Cao C, Li Y (2017) Adsorption of VOCs onto engineered carbon materials: a review. J Hazard Mater 338:102. https://doi.org/10.1016/j.jhazmat.2017.05.013
Ternero-Hidalgo JJ, Rosas JM, Palomo J, Valero-Romero MJ, Rodriguez-Mirasol J, Cordero T (2016) Functionalization of activated carbons by HNO3 treatment: influence of phosphorus surface groups. Carbon 101:409. https://doi.org/10.1016/j.carbon.2016.02.015
Falco C, Marco-Lozar J, Salinas-Torres D, Morallon E, Cazorla-Amoros D, Titirici M (2013) Tailoring the porosity of chemically activated hydrothermal carbons: influence of the precursor and hydrothermal carbonization temperature. Carbon 62:346. https://doi.org/10.1016/j.carbon.2013.06.017
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis 89:143. https://doi.org/10.1016/j.jaap.2010.07.006
Lillo-Rodenas MA, Cazorla-Amoros D, Linares-Solano A (2003) Understanding chemical reactions between carbons and NaOH and KOH an insight into the chemical activation mechanism. Carbon 41:267. https://doi.org/10.1016/S0008-6223(02)00279-8
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition on nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137. https://doi.org/10.1021/es8002684
Han Y, Zhao PP, Dong XT, Zhang C, Liu SX (2014) Improvement in electrochemical capacitance of activated carbon from scrap tires by nitric acid treatment. Front Mater Sci 8(4):391. https://doi.org/10.1007/s11706-014-0271-7
Pak SH, Jeon MJ, Jeon YW (2016) Study of sulfuric acid treatment of activated carbon used to enhance mixed VOC removal. Int Biodeterior Biodegradation 113:195. https://doi.org/10.1016/j.ibiod.2016.04.019
Acknowledgements
This study was supported by the Energy Development Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry and Energy, republic of Korea (Project No. 20162010104680).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kim, JH., Hwang, S.Y., Park, J.E. et al. Impact of the oxygen functional group of nitric acid-treated activated carbon on KOH activation reaction. Carbon Lett. 29, 281–287 (2019). https://doi.org/10.1007/s42823-019-00024-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-019-00024-0