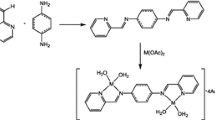
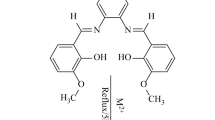
Abstract
In this study, two new Schiff base ligands, (E)-ethyl 2-(2-hydroxybenzylideneamino)-5,5,7,7-tetramethyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylate (L1) and (E)-ethyl 2-(2-hydroxy-3-methoxybenzylideneamino)-5,5,7,7-tetramethyl-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylate (L2), and their corresponding RuII complexes, [L1Ru(p-cymene)]Cl·2.5H2O (1) and [L2RuCl(p-cymene)]1.5H2O (2), were synthesized. The structures of Schiff bases and their RuII‐p‐cymene complexes were elucidated using microanalysis, magnetic susceptibility, molar conductivity, 1H-NMR, 13C-NMR, FT-IR, UV–Vis, LC–MS, and thermogravimetric analysis techniques. Octahedral structures for the obtained RuII‐p‐cymene complexes were proposed. The catalytic activities of the RuII‐p‐cymene complexes obtained after the characterization processes were investigated by using acetophenone derivatives in the transfer hydrogen reactions. The product conversions obtained as a result of catalytic studies were determined using GC–MS. When 4-bromoacetophenone was used as a substrate in the transfer hydrogen reactions, catalysts 1 and 2 showed good catalytic activity of 93% and 96%.
Graphical Abstract





Similar content being viewed by others
References
Rodrigues C, Delolo FG, Ferreira LM, Maia PIS, Deflon VM, Rabeah J, Brückner A, Norinder J, Börner A, Bogado AL, Batista AA (2016) J Mol Struct 1111:84. https://doi.org/10.1016/j.molcata.2016.09.020
Aydemir M, Meric N, Baysal A, Turgut Y, Kayan C, Şeker S, Toğrul M, Gümgüm BJ (2011) Organomet Chem 696:1541–1546. https://doi.org/10.1016/j.jorganchem.2010.12.027
Wu Z, Jiang H (2015) RSC Adv 5:34622. https://doi.org/10.1039/C5RA01893E
Kenny RG, Marmion CJ (2019) Chem Rev 119:1058–1137. https://doi.org/10.1021/acs.chemrev.8b00271
Yang SH, Chang S (2001) Org Lett 3:4209–4211. https://doi.org/10.1021/ol0168768
Murugan K, Vijayapritha S, Kavitha V, Viswanathamurthi P (2020) Polyhedron 190:114737. https://doi.org/10.1016/j.poly.2020.114737
Wang H-X, Wu K, Che C-M (2021) Synlett 32:249–257. https://doi.org/10.1055/s-0040-1707221
Battilocchio C, Hawkins JM, Ley SV (2013) Org Lett 15:2278–2281. https://doi.org/10.1021/ol400856g
Kilbas B, Yilmaz YE, Ergen S (2018) CR Chim 1:880–883. https://doi.org/10.1016/j.crci.2018.07.004
Jia W-G, Zhang H, Zhang T, Xie D, Ling S, Sheng E-H (2016) Organometallics 35(4):503–512. https://doi.org/10.1021/acs.organomet.5b00933
Anh NH, Loi H (2020) Inorg Chem Front 7:583–591
Buldurun K, Turan N, Mahmoudi G, Bursal E (2022) J Mol Struct 1262:133075. https://doi.org/10.1016/j.molstruc.2022.133075
Turan N, Buldurun K, Alan Y, Savci A, Çolak N, Mantarcı A (2019) Res Chem Intermed 45:3525–3540. https://doi.org/10.1007/s11164-019-03806-3
Turan N, Buldurun K, Çolak N, Özdemir İ (2019) Open Chem 17(1):571–580. https://doi.org/10.1515/chem-2019-0074
Akdeniz A (2022) Master Thesis, Natural, and Applied Science, Muş Alparslan University, Muş/Turkey
Sedighipoor M, Kianfara AH, Mohammadnezhad G, Görls H, Plass W (2018) Inorg Chim Acta 476:20–26. https://doi.org/10.1016/j.ica.2018.02.007
Buldurun K, Özdemir M (2020) J Mol Struct 1202:127266. https://doi.org/10.1016/j.molstruc.2019.127266
Turan N, Buldurun K, Adiguzel R, Aras A, Turkan F, Bursal E (2021) J Mol Struct 1244:130989. https://doi.org/10.1016/j.molstruc.2021.130989
Srivastava VK (2021) Future J Pharm Sci 7:51. https://doi.org/10.1186/s43094-021-00191-w
Liu J, Lin Y, Liu M, Wang S, Li Y, Liu X, Tian L (2019) Appl Organomet Chem 33:e4715. https://doi.org/10.1002/aoc.4715
Çolak N, Karayel A, Buldurun K, Turan N (2021) J Struct Chem 62:37–46. https://doi.org/10.1134/S0022476621010054
Han Y-F, Li H, Fei Y, Lin Y-J, Zhang W-Z, Jin G-X (2010) Dalton Trans 39:7119–7124. https://doi.org/10.1039/C0DT00057D
Purkait K, Mukherjee A (2020) Inorg Chim Acta 502:119361. https://doi.org/10.1016/j.ica.2019.119361
Alomar K, Khan MA, Allain M, Bouet G (2009) Polyhedron 28:1273–1280. https://doi.org/10.1016/j.poly.2009.02.042
Salavati-Niasari M, Davar F, Saberyan K (2010) Polyhedron 29(10):2149–2156. https://doi.org/10.1016/j.poly.2010.04.003
El-Gammal OA, Elmorsy EA, Sherif YE (2014) Spectrochim Acta A 120:332–339. https://doi.org/10.1016/j.saa.2013.09.067
Starha P, Travnicek Z, Krikavova R, Dvorak Z (2016) Molecules 21:1725. https://doi.org/10.3390/molecules21121725
Pandiarajan D, Ramesh R (2013) J Organomet Chem 723:26–35. https://doi.org/10.1016/j.jorganchem.2012.10.003
Ramesh M, Venkatachalam G (2019) J Organomet Chem 880:47–55. https://doi.org/10.1016/j.jorganchem.2018.10.029
Acknowledgements
The authors extent their appreciation to the Muş Alparslan University Scientific Research Projects Center (BAP) under research project no BAP-20-FEF-4902-06 for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turan, N., Akdeniz, A. Synthesis, Structural Characterization of Schiff Base Ligands and Their RuII‐p‐Cymene Complexes, and Catalytic Activity in the Transfer Hydrogenation of Ketones. Catal Lett 153, 3009–3018 (2023). https://doi.org/10.1007/s10562-022-04222-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04222-w