Abstract
A highly efficient heterogeneous copper(I)-catalyzed decarboxylative C–C coupling of α-amino acids with various carbon nucleophiles has been developed that proceeds smoothly in toluene at 110 °C by using an 2-aminoethylamino-modified SBA-15-supported copper(I) bromide complex as the catalyst and di-tert-butyl peroxide as the oxidant and provides a novel and practical approach for the synthesis of diverse propargylamines, indolyl pyrrolidines and piperidines, and β-nitroamines in good yields. The new heterogeneous copper(I) catalyst can be facilely prepared from commercially easily available and inexpensive reagents via a simple procedure, and exhibits a slightly higher catalytic activity than homogeneous CuBr/TMEDA system and can be recycled at least 8 times without any apparent loss of catalytic efficiency.
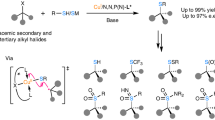
Graphical Abstract
A recyclable copper(I)-catalyzed decarboxylative C–C coupling of α-amino acids with various carbon nucleophiles has been developed by using an SBA-15-supported copper(I) bromide complex as the catalyst, providing a novel and practical approach for the synthesis of diverse propargylamines, indolyl pyrrolidines and piperidines, and β-nitroamines.








Similar content being viewed by others
References
Vollhardt KPC, Schore NE (2000) Organische Chemie, 3rd edn. Wiley, Weinheim, p 1081
Gooßen LJ, Rodriguez N, Gooßen K (2008) Angew Chem Int Ed 47:3100–3120
Rodriguez N, Gooßen LJ (2011) Chem Soc Rev 40:5030–5048
Weaver JD, Recio A, Grenning AJ, Tunge JA (2011) Chem Rev 111:1846–1913
Patra T, Maiti D (2017) Chem Eur J 23:7382–7401
Wei Y, Hu P, Zhang M, Su W (2017) Chem Rev 117:8864–8907
Yin F, Wang Z, Li Z, Li C (2012) J Am Chem Soc 134:10401–10404
Kong D, Moon PJ, Bsharat O, Lundgren RJ (2020) Angew Chem Int Ed 59:1313–1319
Edwards JT, Merchant RR, McClymont KS, Knouse KW, Qin T, Malins LR, Vokits B, Shaw SA, Bao D-H, Wei F-L, Zhou T, Eastgate MD, Baran PS (2017) Nature 545:213–218
Ventre S, Petronijevic FR, MacMillan DWC (2015) J Am Chem Soc 137:5654–5657
Zhao W, Wurz RP, Peters JC, Fu GC (2017) J Am Chem Soc 139:12153–12156
Liang Y, Zhang X, MacMillan DWC (2018) Nature 559:83–88
Fu M-C, Shang R, Zhao B, Wang B, Fu Y (2019) Science 363:1429–1434
Na CG, Ravelli D, Alexanian EJ (2020) J Am Chem Soc 142:44–49
Saxton JE (1997) Nat Prod Rep 14:559–590
Mitchenson A, Nadin A (2000) J Chem Soc Perkin Trans 1:2862–2892
Cox ED, Cook JM (1995) Chem Rev 95:1797–1842
Le C, Liang Y, Evans RW, Li X, MacMillan DWC (2017) Nature 547:79–83
Trowbridge A, Reich D, Gaunt MJ (2018) Nature 561:522–527
Paul A, Seidel D (2019) J Am Chem Soc 141:8778–8782
Li C-J (2009) Acc Chem Res 42:335–344
Girard SA, Knauber T, Li C-J (2014) Angew Chem Int Ed 53:74–100
Bi H-P, Zhao L, Liang Y-M, Li C-J (2009) Angew Chem Int Ed 48:792–795
Bi HP, Teng Q, Guan M, Chen WW, Liang YM, Yao X, Li CJ (2010) J Org Chem 75:783–788
Zhang C, Seidel D (2010) J Am Chem Soc 132:1798–1799
Kumar A, Kumar M, Gupta LP, Gupta MK (2014) RSC Adv 4:9412–9415
Mao Z-Y, Liu Y-W, Ma R-J, Ye J-L, Si C-M, Wei B-G, Lin G-Q (2019) Chem Commun 55:14170–14173
Noble A, MacMillan DWC (2014) J Am Chem Soc 136:11602–11605
Zou Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC (2014) Science 345:437–440
Jiang M, Yang H, Fu H (2016) Org Lett 18:1968–1971
Zhang H, Zhang P, Jiang M, Yang H, Fu H (2017) Org Lett 19:1016–1019
Li J, Lefebvre Q, Yang H, Zhao Y, Fu H (2017) Chem Commun 53:10299–10302
Guo J, Xie Y, Wu Q-L, Zeng W-T, Chan ASC, Weng J, Lu G (2018) RSC Adv 8:16202–16206
Bi H-P, Chen W-W, Liang Y-M, Li C-J (2009) Org Lett 11:3246–3249
Gladysz JA (2009). In: Benaglia M (ed) Recoverable and recyclable catalysts. Wiley, Chichester, pp 1–14
Macquarrie D (2010). In: Barbaro P, Liguori F (eds) Heterogenized homogeneous catalysts for fine chemicals production. Springer, Berlin, pp 1–35
Phan NTS, Sluys MVD, Jones CW (2006) Adv Synth Catal 348:609–679
Akiyama R, Kobayashi S (2009) Chem Rev 109:594–642
Climent MJ, Corma A, Iborra S (2011) Chem Rev 111:1072–1133
Benyahya S, Monnier F, Taillefer M, Chi Man MW, Bied C, Ouazzani F (2008) Adv Synth Catal 350:2205–2208
Benyahya S, Monnier F, Chi Man MW, Bied C, Ouazzani F, Taillefer M (2009) Green Chem 11:1121–1123
Phan NTS, Nguyen TT, Nguyen VT, Nguyen KD (2013) ChemCatChem 5:2374–2379
Yang J, Li P, Wang L (2011) Tetrahedron 67:5543–5549
Magne V, Garnier T, Danel M, Pale P, Chassaing S (2015) Org Lett 17:4494–4497
Zhao H, Jiang Y, Chen Q, Cai M (2015) New J Chem 39:2106–2115
Niu B, You C, Huang B, Cai M (2019) Catal Commun 123:11–16
Kantam ML, Reddy CV, Srinivas P, Bhargava S (2013) In Topics in Organometallic Chemistry, Eds. Taillefer M, Ma D, Springer, Heidelberg, vol. 46, pp.119
Chassaing S, Sido ASS, Alix A, Kumarraja M, Pale P, Sommer J (2008) Chem Eur J 14:6713–6721
Pan S, Yan S, Osako T, Uozumi Y (2017) ACS Sustain Chem Eng 5:10722–10734
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Science 279:548–552
Friedrich H, Sietsma JRA, de Jongh PE, Verkleij AJ, de Jong KP (2007) J Am Chem Soc 129:10249–10254
Baltes M, Cassiers K, Van Der Voort P, Weckhuysen BM, Schoonheydt RA, Vansant EF (2001) J Catal 197:160–171
Rohani S, Ziarani GM, Badiei A, Ziarati A, Jafari M, Shayyesteh A (2018) Appl Organometall Chem 32:e4397
Nouri K, Hajjami M, Azadi G (2018) Catal Lett 148:671–679
Lai YT, Chen TC, Lan YK, Chen BS, You JH, Yang CM, Lai NC, Wu JH, Chen CS (2014) ACS Catal 4:3824–3836
Huo Y, Hu J, Lin S, Ju X, Wei Y, Huang Z, Hu Y, Tu Y (2019) Appl Organometall Chem 33:e4874
Zhao J, Yuan H, Qin X, Tian K, Liu Y, Wei C, Zhang Z, Zhou L, Fang S (2020) Catal Lett 150:2841–2849
Chen CS, Lai YT, Lai TW, Wu JH, Chen CH, Lee JF, Kao HM (2013) ACS Catal 3:667–677
Bardajee GR, Mohammadi M, Kakavand N (2016) Appl Organometall Chem 30:51–58
Hajjami M, Ghorbani F, Yousofvand Z (2017) Appl Organometall Chem 31:3843
Luan Z, Fournier JA (2005) Micro Meso Mater 79:235–240
Lucet D, Le Gall T, Mioskowski C (1998) Angew Chem Int Ed 37:2580–2627
Lempers HEB, Sheldon RA (1998) J Catal 175:62–69
Acknowledgements
We thank the National Natural Science Foundation of China (No. 21664008) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author guarantees that there is no conflict of interest with the co-author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, R., Li, J., Huang, B. et al. Recyclable Copper-Catalyzed Decarboxylative C–C Coupling of the sp3-Hybridized Carbon Atoms of α-Amino Acids. Catal Lett 153, 178–187 (2023). https://doi.org/10.1007/s10562-022-03936-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-03936-1