Abstract
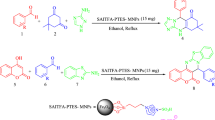
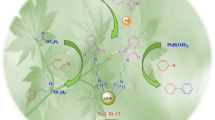
Organocatalysts, like a minimalistic biocatalyst, pursue to reduce metal consumption with low cost, and low toxicity targeting to become a green strategy in organic transformations. Supporting organocatalyst on magnetite nanoparticles has been attracted researchers’ attention in recent years due to the facilitating catalyst recycling and recovery procedure. However, the industrial organocatalysts design strategy limits the recyclability and the practical usability of these catalysts. Here, we present a facile and novel protocol for camphor sulfonic acid immobilization onto 3-aminopropyltriethoxysilane-modified magnetic nanoparticles under mild reaction conditions. The magnetic organocatalyst is characterized by different techniques such as FT-IR, XRD, TGA, SEM, and EDS. Recyclable magnetic camphor sulfonic acid-catalyzed practical and regioselective one-pot, multicomponent synthesis of 2,3-diphenyl-2,3-dihydro-4H-1,3-benzothiazin-4-one derivatives in polyethylene glycol (PEG-200) for 120–240 min in good to excellent yields. Not only the novel catalyst gets the reaction to gain a high yield, but also it is reusable at least 5 times the reaction cycle by applying an external magnetic field to get overall sustainable process development.
Graphical abstract












Similar content being viewed by others
References
Peng L, Zhang J, Yang S, Han B, Sang X, Liu C et al (2015) Ultra-small gold nanoparticles immobilized on mesoporous silica/graphene oxide as highly active and stable heterogeneous catalysts. Chem Commun 51(21):4398–4401
Azarkamanzad Z, Farzaneh F, Maghami M, Simpson J (2019) Synthesis, crystal structure and immobilization of a new cobalt (ii) complex with a 2, 4, 6-tris (2-pyridyl)-1, 3, 5-triazine ligand on modified magnetic nanoparticles as a catalyst for the oxidation of alkanes. New J Chem 43(30):12020–12031
Hajibeygi M, Habibnejad N, Shabanian M, Khonakdar HA (2021) Fabrication and study of thermal and combustion resistance of DOPO-functionalized polyamide reinforced with organo-modified Mg(OH)2 nanoparticles. Polym Int 70(3):317–330
Karimi B, Ghaffari B, Vali H (2021) Synergistic catalysis within core-shell Fe3O4@SiO2 functionalized with triethylene glycol (TEG)-imidazolium ionic liquid and tetramethylpiperidine N-oxyl (TEMPO) boosting selective aerobic oxidation of alcohols. J Colloid Interface Sci 589:474–485
Chen M-N, Mo L-P, Cui Z-S, Zhang Z-H (2019) Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr Opin Green Sustain Chem 15:27–37
Gao G, Di J-Q, Zhang H-Y, Mo L-P, Zhang Z-H (2020) A magnetic metal organic framework material as a highly efficient and recyclable catalyst for synthesis of cyclohexenone derivatives. J Catal 387:39–46
Yamaguchi Y, Aono R, Hayashi E, Kamata K, Hara M (2020) Template-free synthesis of mesoporous β-MnO2 nanoparticles: structure, formation mechanism, and catalytic properties. ACS Appl Mater Interfaces 12(32):36004–36013
Gu Y, Son SU, Li T, Tan B (2021) Low-cost hypercrosslinked polymers by direct knitting strategy for catalytic applications. Adv Func Mater 31(12):2008265
Zhang S, Zhao X, Niu H, Shi Y, Cai Y, Jiang G (2009) Superparamagnetic Fe3O4 nanoparticles as catalysts for the catalytic oxidation of phenolic and aniline compounds. J Hazard Mater 167(1–3):560–566
Devi V, Selvaraj M, Selvam P, Kumar AA, Sankar S, Dinakaran K (2017) Preparation and characterization of CNSR functionalized Fe3O4 magnetic nanoparticles: an efficient adsorbent for the removal of cadmium ion from water. J Environ Chem Eng 5(5):4539–4546
Wu R, Liu J-H, Zhao L, Zhang X, Xie J, Yu B et al (2014) Hydrothermal preparation of magnetic Fe3O4@C nanoparticles for dye adsorption. J Environ Chem Eng 2(2):907–913
Xu J-K, Zhang F-F, Sun J-J, Sheng J, Wang F, Sun M (2014) Bio and nanomaterials based on Fe3O4. Molecules 19(12):21506–21528
Kim SB, Cai C, Kim J, Sun S, Sweigart DA (2009) Surface Modification of Fe3O4 and FePt magnetic nanoparticles with organometallic complexes. Organometallics 28(18):5341–5348
Zhang M, Liu Y-H, Shang Z-R, Hu H-C, Zhang Z-H (2017) Supported molybdenum on graphene oxide/Fe3O4: an efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal Commun 88:39–44
Tang NJ, Zhong W, Jiang HY, Wu XL, Liu W, Du YW (2004) Nanostructured magnetite (Fe3O4) thin films prepared by sol–gel method. J Magn Magn Mater 282:92–95
Liang X, Jia X, Cao L, Sun J, Yang Y (2010) Microemulsion synthesis and characterization of nano-Fe3O4 particles and Fe3O4 nanocrystalline. J Dispersion Sci Technol 31(8):1043–1049
Kumar P, Gupta P, Sharma C (2021) Surface modified novel magnetically tuned halloysite functionalized sulfonic acid: synthesis, characterization and catalytic activity. Catal Sci Technol 11:3775–3786
Mahajan A, Gupta P (2020) Halloysite nanotubes based heterogeneous solid acid catalysts. New J Chem 44(30):12897–12908
Brahmachari G, Nurjamal K, Karmakar I, Mandal M (2018) Camphor-10-sulfonic acid (CSA): a water compatible organocatalyst in organic transformations. Current Organocatalysis 5(3):165–181
Banerjee B (2017) Recent developments on ultrasound-assisted one-pot multicomponent synthesis of biologically relevant heterocycles. Ultrason Sonochem 35:15–35
Eicher T, Hauptmann S, Speicher A (2013) The chemistry of heterocycles: structures, reactions, synthesis, and applications. Wiley, New York
Koketsu M, Tanaka K, Takenaka Y, Kwong CD, Ishihara H (2002) Synthesis of 1,3-thiazine derivatives and their evaluation as potential antimycobacterial agents. Eur J Pharm Sci 15(3):307–310
Badshah SL, Naeem A (2016) Bioactive thiazine and benzothiazine derivatives: green synthesis methods and their medicinal importance. Molecules 21(8):1054
Brahmachari G, Banerjee B (2014) Facile and one-pot access to diverse and densely functionalized 2-amino-3-cyano-4 H-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalyst. ACS Sustain Chem Eng 2(3):411–422
Jana S, Ghosh SK, Nath S, Pande S, Praharaj S, Panigrahi S et al (2006) Synthesis of silver nanoshell-coated cationic polystyrene beads: a solid phase catalyst for the reduction of 4-nitrophenol. Appl Catal A 313(1):41–48
Chakraborti AK, Rudrawar S, Jadhav KB, Kaur G, Chankeshwara SV (2007) “On water” organic synthesis: a highly efficient and clean synthesis of 2-aryl/heteroaryl/styryl benzothiazoles and 2-alkyl/aryl alkyl benzothiazolines. Green Chem 9(12):1335–1340
Chakraborti AK, Selvam C, Kaur G, Bhagat S (2004) An efficient synthesis of benzothiazoles by direct condensation of carboxylic acids with 2-aminothiophenol under microwave irradiation. Synlett 2004(05):0851–0855
Kumar D, Sonawane M, Pujala B, Jain VK, Bhagat S, Chakraborti AK (2013) Supported protic acid-catalyzed synthesis of 2, 3-disubstituted thiazolidin-4-ones: enhancement of the catalytic potential of protic acid by adsorption on solid supports. Green Chem 15(10):2872–2884
Gao X, Yu B, Yang Z, Zhao Y, Zhang H, Hao L et al (2015) Ionic liquid-catalyzed C-S bond construction using CO2 as a C1 building block under mild conditions: a metal-free route to synthesis of benzothiazoles. ACS Catal 5(11):6648–6652
Liu Z, Zhang J, Han B (2005) Chemical reactions in Super2 and subcritical water. Progress Chem 17(02):266
Yazdani F, Fattahi B, Azizi N (2016) Synthesis of functionalized magnetite nanoparticles to use as liver targeting MRI contrast agent. J Magn Magn Mater 406:207–211
Shahabi SS, Azizi N, Vatanpour V (2019) Synthesis and characterization of novel g-C3N4 modified thin film nanocomposite reverse osmosis membranes to enhance desalination performance and fouling resistance. Sep Purif Technol 215:430–440
Azizi N, Ahooie TS, Hashemi MM (2017) Multicomponent domino reactions in deep eutectic solvent: an efficient strategy to synthesize multisubstituted cyclohexa-1,3-dienamines. J Mol Liq 246:221–224
Hosseini M-S, Masteri-Farahani M (2020) Fabrication of new magnetite based sulfonic-phosphotungstic dual-acid catalyst for catalytic acetalization of benzaldehyde with ethylene glycol. React Kinet Mech Catal 130(2):979–991
Ni X, Zheng Z, Hu X, Xiao X (2010) Silica-coated iron nanocubes: preparation, characterization and application in microwave absorption. J Colloid Interface Sci 341(1):18–22
Yang J, Tan J-N, Gu Y (2012) Lactic acid as an invaluable bio-based solvent for organic reactions. Green Chem 14(12):3304–3317
Silverberg LJ, Pacheco C, Lagalante A, Tierney J, Bachert JT, Bayliff JA et al (2016) Synthesis and spectroscopic properties of a series of novel 2-aryl-3-phenyl-2, 3-dihydro-4H-1, 3-benzothiazin-4-ones. ARKIVOC 1:S43
Zhou B, Yang J, Li M, Gu Y (2011) Gluconic acid aqueous solution as a sustainable and recyclable promoting medium for organic reactions. Green Chem 13(8):2204–2211
Acknowledgements
Financial support of this work by Iran National Science Foundation (INSF, Grant Number: 98015375) is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azizi, N., Farzaneh, F. & Habibnejad, N. Recyclable Magnetic Camphor Sulfonic Acid: A Reliable and Highly Efficient Ionic Organocatalyst for Benzothiazin-4-One Synthesis in Green Media. Catal Lett 152, 3146–3157 (2022). https://doi.org/10.1007/s10562-021-03885-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03885-1