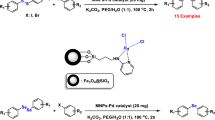
Abstract
This fact that diaryl sulfides are important structural components in polymers, agrochemicals, natural products, and pharmaceutical intermediates has increased the attention of chemists to synthesize these compounds. In recent times, the research on the preparation of diaryl sulfides using magnetically reusable catalysts have received profound attention in organic chemistry. In this paper, we describe the fabrication and characterization of a magnetically reusable palladium nanomaterial and evaluate its catalytic activity for the preparation of diaryl sulfides through one-pot three-component coupling reaction of azoles, S8 and aryl iodides. The structure of palladium supported on magnetic nanoparticles was well characterized by a series of spectroscopic techniques including FT-IR, SEM, TEM, EDX, XRD, VSM, TGA, ICP-OES. To the best of our knowledge, it is the first report on the utilization of Pd nanomagnetic catalyst for the one-pot three-component coupling reaction of azoles, S8 and aryl iodides.
Graphical Abstract










Similar content being viewed by others
Change history
30 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10562-023-04278-2
References
Shakeri M, Shal ZK, Van Der Voort P (2021) An overview of the challenges and progress of synthesis, characterization and applications of plugged sba-15 materials for heterogeneous catalysis. Materials (Basel). https://doi.org/10.3390/ma14175082
Hunt AJ, Anderson CWN, Bruce N et al (2014) Phytoextraction as a tool for green chemistry. Green Process Synth 3:3–22. https://doi.org/10.14315/evth-2014-0205
Govan J, Gun’ko YK (2014) Recent advances in the application of magnetic nanoparticles as a support for homogeneous catalysts. Nanomaterials 4:222–241. https://doi.org/10.3390/nano4020222
Maroa S, Inambao F (2021) A review of sustainable biodiesel production using biomass derived heterogeneous catalysts. Eng Life Sci 21:790–824. https://doi.org/10.1002/elsc.202100025
Docherty SR, Rochlitz L, Payard PA, Copéret C (2021) Heterogeneous alkane dehydrogenation catalysts investigated via a surface organometallic chemistry approach. Chem Soc Rev 50:5806–5822. https://doi.org/10.1039/d0cs01424a
Maleki M, Baghbanian SM, Tajbakhsh M (2018) Heteropolyacid immobilized on polymer/magnetic zeolite nanocomposite as a new and recyclable catalyst for the selective oxidation of alcohols. J Iran Chem Soc 15:359–368. https://doi.org/10.1007/s13738-017-1237-3
Kim DS, Park WJ, Jun CH (2017) Metal-organic cooperative catalysis in C-H and C-C bond activation. Chem Rev 117:8977–9015. https://doi.org/10.1021/acs.chemrev.6b00554
Narimani H (2022) Research on synthesis of heterocyclic structures using ZnO NPs as catalyst. J Synth Chem 1:62–83. https://doi.org/10.22034/jsc.2022.155232
Arabmarkadeh A, Javahershenas R, Kazemi M (2021) Nanomaterials: catalysis in synthesis of highly substituted heterocycles. Synth Commun 51:880–903. https://doi.org/10.1080/00397911.2020.1864646
Atashkar B, Zolfigol MA, Mallakpour S (2018) Applications of biological urea-based catalysts in chemical processes. Mol Catal 452:192–246. https://doi.org/10.1016/j.mcat.2018.03.009
Nair PP, Philip RM, Anilkumar G (2021) Nickel catalysts in Sonogashira coupling reactions. Org Biomol Chem 19:4228–4242. https://doi.org/10.1039/D1OB00280E
Pawar A, Gajare S, Jagdale A et al (2022) Supported NHC-benzimi@Cu complex as a magnetically separable and reusable catalyst for the multicomponent and click synthesis of 1,4-disubstituted 1,2,3-triazoles via huisgen 1,3-dipolar cycloaddition. Catal Lett 152:1854–1868. https://doi.org/10.1007/s10562-021-03772-9
Niknam K, Saberi D, Molaee H, Zolfigol MA (2010) Silica-bonded S-sulfonic acid as a recyclable catalyst for the silylation of hydroxyl groups with hexamethyldisilazane (HMDS). Can J Chem 88:164–171. https://doi.org/10.1139/V09-162
Wen C, Yin A, Dai W-L (2014) Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl Catal B Environ 160–161:730–741. https://doi.org/10.1016/j.apcatb.2014.06.016
De S, Dokania A, Ramirez A, Gascon J (2020) Advances in the design of heterogeneous catalysts and thermocatalytic processes for CO2Utilization. ACS Catal 10:14147–14185. https://doi.org/10.1021/acscatal.0c04273
Karakhanov E, Maximov A, Zolotukhina A (2022) Heterogeneous dendrimer-based catalysts. Polymers (Basel) 14:981. https://doi.org/10.3390/polym14050981
Mirzaee M, Bahramian B, Mirebrahimi M (2016) Amine-functionalized boehmite nanoparticle-supported molybdenum and vanadium complexes: efficient catalysts for epoxidation of alkenes. Cuihua Xuebao/Chinese J Catal 37:1263–1274. https://doi.org/10.1016/S1872-2067(16)62451-8
Verma M, Mandyal P, Singh D, Gupta N (2020) Recent developments in heterogeneous catalytic routes for the sustainable production of succinic acid from biomass resources. Chemsuschem 13:4026–4034. https://doi.org/10.1002/cssc.202000690
Yilmaz B, Trukhan N, Müller U (2012) Industrial outlook on zeolites and metal organic frameworks. Cuihua Xuebao/Chinese J Catal 33:3–10. https://doi.org/10.1016/s1872-2067(10)60302-6
Baron R, Wildgoose GG, Compton RG (2009) Metallic nanoparticles deposited on carbon microspheres: novel materials for combinatorial electrochemistry and electroanalysis. J Nanosci Nanotechnol 9:2274–2282. https://doi.org/10.1166/jnn.2009.SE14
Lu C, Niu X, Zhang W et al (2020) Iron nanoparticles loaded on nickel sulfide nanosheets: an efficient amorphous catalyst for water oxidation. Sustain Energy Fuels 4:5498–5502. https://doi.org/10.1039/d0se01088j
Bodaghifard MA, Hamidinasab M, Ahadi N (2018) Recent advances in the preparation and application of organic– inorganic hybrid magnetic nanocatalysts on multicomponent reactions. Curr Org Chem 22:234–267. https://doi.org/10.2174/1385272821666170705144854
Heravi MM, Mohammadi P (2022) Layered double hydroxides as heterogeneous catalyst systems in the cross-coupling reactions: an overview. Mol Divers 26:569–587. https://doi.org/10.1007/s11030-020-10170-7
Abedi M, Hosseini M, Arabmarkadeh A, Kazemi M (2021) Magnetic nanocatalysts in A3-coupling reactions. Synth Commun 51:835–855. https://doi.org/10.1080/00397911.2020.1858320
Lu F, Astruc D (2020) Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2020.213180
Bhalothia D, Krishnia L, Yang S-S et al (2020) Recent advancements and future prospects of noble metal-based heterogeneous nanocatalysts for oxygen reduction and hydrogen evolution reactions. Appl Sci 10:1–19. https://doi.org/10.3390/app10217708
Chen MN, Mo LP, Cui ZS, Zhang ZH (2019) Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr Opin Green Sustain Chem 15:27–37. https://doi.org/10.1016/j.cogsc.2018.08.009
Sharma A, Wakode S, Sharma S et al (2020) Methods and strategies used in green chemistry: a review. Curr Org Chem 24:2555–2565. https://doi.org/10.2174/1385272824999200802025233
Vogt C, Weckhuysen BM (2022) The concept of active site in heterogeneous catalysis. Nat Rev Chem 6:89–111. https://doi.org/10.1038/s41570-021-00340-y
Xu Y, Cao M, Zhang Q (2021) Recent advances and perspective on heterogeneous catalysis using metals and oxide nanocrystals. Mater Chem Front 5:151–222. https://doi.org/10.1039/d0qm00549e
Chen X, Qian D, Xu G et al (2019) Magnetic Fe3O4 supported PdAu bimetallic nanoparticles with the enhanced catalytic activity for Heck and Suzuki cross-coupling reactions. Colloids Surfaces A Physicochem Eng Asp 573:67–72. https://doi.org/10.1016/j.colsurfa.2019.04.013
Krishnan SG, Pua F-L, Zhang F (2021) A review of magnetic solid catalyst development for sustainable biodiesel production. Biomass Bioenerg. https://doi.org/10.1016/j.biombioe.2021.106099
Sun C, Zhou R, Jianan E et al (2016) Ascorbic acid-coated Fe3O4 nanoparticles as a novel heterogeneous catalyst of persulfate for improving the degradation of 2,4-dichlorophenol. RSC Adv 6:10633–10640. https://doi.org/10.1039/c5ra22491h
Korany MA, Mahgoub H, Haggag RS et al (2017) Green chemistry: analytical and chromatography. J Liq Chromatogr Relat Technol 40:839–852. https://doi.org/10.1080/10826076.2017.1373672
Sadeghpour M, Olyaei A (2021) Recent advances in the synthesis of bis(pyrazolyl)methanes and their applications. Res Chem Intermed 47:4399–4441. https://doi.org/10.1007/s11164-021-04592-7
Govardhana Reddy PV, Rajendra Prasad Reddy B, Venkata Krishna Reddy M et al (2021) A review on multicomponent reactions catalysed by zero-dimensional/one-dimensional titanium dioxide (TiO2) nanomaterials: promising green methodologies in organic chemistry. J Environ Manage 279:111603. https://doi.org/10.1016/j.jenvman.2020.111603
Singh MS, Chowdhury S (2012) Recent developments in solvent-free multicomponent reactions: a perfect synergy for eco-compatible organic synthesis. RSC Adv 2:4547–4592. https://doi.org/10.1039/C2RA01056A
Marson CM (2012) Multicomponent and sequential organocatalytic reactions: diversity with atom-economy and enantiocontrol. Chem Soc Rev 41:7712–7722. https://doi.org/10.1039/c2cs35183h
Sinha SK, Panja S, Grover J et al (2022) Dual Ligand Enabled Nondirected C-H Chalcogenation of Arenes and Heteroarenes. J Am Chem Soc 144:12032–12042. https://doi.org/10.1021/jacs.2c02126
Cai M, Yao R, Chen L, Zhao H (2014) A simple, efficient and recyclable catalytic system for carbon-sulfur coupling of aryl halides with thioacetamide. J Mol Catal A Chem 395:349–354. https://doi.org/10.1016/j.molcata.2014.08.010
Farzin S, Rahimi A, Amiri K et al (2018) Synthesis of diaryl sulfides via nickel ferrite-catalysed C─S bond formation in green media. Appl Organomet Chem 32:e4409. https://doi.org/10.1002/aoc.4409
Lamani DS, Badiger SG, Singh M (2019) Formation of diaryl sulfides and sulfoxides: Synthesis, characterization and structure activity correlation studies. Phosphorus, Sulfur Silicon Relat Elem 194:789–795. https://doi.org/10.1080/10426507.2018.1547720
Vásquez-Céspedes S, Ferry A, Candish L, Glorius F (2015) Heterogeneously catalyzed direct C-H thiolation of heteroarenes. Angew Chemie 54:5772–5776. https://doi.org/10.1002/anie.201411997
Reddy KHV, Prakash VR, Kumar AA et al (2011) Nano copper oxide catalyzed synthesis of symmetrical diaryl sulfides under ligand free conditions. Beilstein J Org Chem 7:886–891. https://doi.org/10.3762/bjoc.7.101
Gilman H, Smith Broadbent H (1947) Some basically substituted diaryl sulfides and sulfones. J Am Chem Soc 69:2053–2057. https://doi.org/10.1021/ja01200a069
Kandeel MM, Youssef MSK (2005) The utility of diaryl sulfides and diaryl sulfones in heterocyclic synthesis [1993–2003]. Phosphorus, Sulfur Silicon Relat Elem 180:217–282. https://doi.org/10.1080/104265090508541
Gogoi P, Gogoi SR, Kalita M, Barman P (2013) Silver ion mediated in situ synthesis of mixed diaryl sulfides from diaryl disulfides. Synlett 24:873–877. https://doi.org/10.1055/s-0032-1318482
Rostami A, Rostami A, Iranpoor N, Zolfigol MA (2016) Efficient Cu-catalyzed one-pot odorless synthesis of sulfides from triphenyltin chloride, aryl halides and S8 in PEG. Tetrahedron Lett 57:192–195. https://doi.org/10.1016/j.tetlet.2015.11.093
Jasim SA, Riadi Y, Majdi HS, Altimari US (2022) Nanomagnetic macrocyclic Schiff-base-Mn(ii) complex: an efficient heterogeneous catalyst for click approach synthesis of novel β-substitued-1,2,3-triazoles. RSC Adv 12:17905–17918. https://doi.org/10.1039/d2ra02587f
Maleki B, Reiser O, Esmaeilnezhad E, Choi HJ (2019) SO3H-dendrimer functionalized magnetic nanoparticles (Fe3O4@D–NH–(CH2)4–SO3H): Synthesis, characterization and its application as a novel and heterogeneous catalyst for the one-pot synthesis of polyfunctionalized pyrans and polyhydroquinolines. Polyhedron 162:129–141. https://doi.org/10.1016/j.poly.2019.01.055
Topal U, Aksan MA (2016) Phase stabilization of magnetite (Fe3O4) nanoparticles with B2O3 addition: a significant enhancement on the phase transition temperature. J Magn Magn Mater 406:123–128. https://doi.org/10.1016/j.jmmm.2016.01.007
Ranjit S, Lee R, Heryadi D et al (2011) Copper-mediated C-H activation/C–S cross-coupling of heterocycles with thiols. J Org Chem 76:8999–9007. https://doi.org/10.1021/jo2017444
Inomata H, Toh A, Mitsui T, Fukuzawa S (2013) N-heterocyclic carbene copper(I) complex-catalyzed direct C-H thiolation of benzothiazoles. Tetrahedron Lett 54:4729–4731. https://doi.org/10.1016/j.tetlet.2013.06.104
Arisawa M, Toriyama F, Yamaguchi M (2011) Rhodium-catalyzed phenylthiolation reaction of heteroaromatic compounds using α-(phenylthio)isobutyrophenone. Tetrahedron Lett 52:2344–2347. https://doi.org/10.1016/j.tetlet.2011.02.077
Panda N, Jena AK, Mohapatra S (2012) Heterogeneous magnetic catalyst for S-arylation reactions. Appl Catal A Gen 433–434:258–264. https://doi.org/10.1016/j.apcata.2012.05.026
Acknowledgements
Open fund project of Hubei Key Laboratory of Kidney Disease Pathogenesis and Intervention (SB202120), Scientific research project of Hubei Provincial Health Commission (WJ2021F028), Scientific research project of Hubei Polytechnic University (22XJZ04Y), Hubei University laboratory research project (HBSY2019-36).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: email address of Jin Liu has been updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Jin, S., Qin, S. et al. 15-Membered Macrocyclic Schiff-Base-Pd(0) Complex Immobilized on Fe3O4 MNPs: An Novel Nanomagnetic Catalyst for the One-Pot Three-Component C–H Chalcogenation of Azoles by S8 and Aryl Iodides. Catal Lett 153, 2581–2591 (2023). https://doi.org/10.1007/s10562-022-04194-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04194-x