Abstract
The subject is a debate on the formation mechanism of the environmentally persistent free radicals (EPFR), by interaction of aromatics with transition metal oxides. Literature reports contrasting opinions concerning the stabilization of the phenoxyradicals mainly as concerns the suggested reduction of interacting transition metal centers. The present paper, in accordance with the mechanisms assessed by a number of spectroscopic and computational results, excludes the necessity that the catalytic mechanism involves the reduction of the metal centers by interaction with the aromatics.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Combustion and thermal processes produce severe toxic components, like particulate matter, polychlorinated dibenzo-p-dioxins, polycyclic and aromatic hydrocarbons, environmentally persistent free radicals (EPFR). These radicals are thought to be generated by interaction of aromatic compounds with the environmental transition metal oxides (iron, copper, zinc, nickel oxides) [1, 2] and are expected to produce reactive oxygen species (ROS) like OH●, O●2−, HO2● HO2●−, H2O2 [3]. ROS are able to induce biological damages, going from inflammation to serious DNA damage, hence the relevance of acknowledging the mechanism of radical formation and in particular the catalytic role of the transition metal ions.
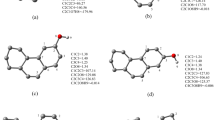
In the last 10 years the mechanism proposed by Dellinger et al. [1, 2] was dominating all the investigations. It has been suggested that firstly the molecular aromatic precursor chemisorbs on the oxide surface, with elimination of water, then an electron transfer from the aromatic molecule to the transition metal center takes place and causes both reduction of the metal and EPFR generation (scheme 1a). The above mechanism [4] has been assessed detecting the radical species by the electron spin resonance (ESR); however the same ESR technique did not give sure evidence of the reduction of the transition metal center.
a Dellinger mechanism [4] for the formation of phenoxyradical. b Cosentino mechanism [5] for the formation of phenoxyradical. In the reaction scheme also the relative electron energies of intermediates with respect to separate reagents are reported (in round parentheses kcal/mol) as well as the activation energies (in square parentheses kcal/mol). For the A5 intermediate, which is very stable with respect to separate reagents, a biradical singlet may be hypothesized featuring the antiferromagnetic coupling that quenches the ESR lines. The mechanism is independent from the presence of the OH group in the aromatic molecule
On the other hand the spectroscopic and computational model proposed by Cosentino, Morazzoni et al. [5], gives evidence of a mechanism triggered by O2 interaction, where the decrease in intensity of the Cu(II) ESR lines was attributed to antiferromagnetic interaction between the phenoxyl radical and the Cu(II) center (Scheme 1b, species A5), instead of the Cu(II) reduction proposed by Dillinger.
More recently different authors investigated the formation of EPFR radicals on several oxides; the abilities of the metal oxides to promote EPFR formation were in the order Al2O3 > ZnO > CuO > NiO [6], in particular the radicals are most stable on ZnO. This is a highly ubiquitous oxide where the radical longevity may present an ongoing environmental hazard. Zn2+centers cannot be significantly reduced to Zn+ under contact by aromatics, thus the Dellinger’s mechanism becomes even more questionable in this case. Very recently an investigation of the electronic state of ZnO after chemisorption of the aromatic molecule demonstrated, through photoelectron spectroscopy (XPS) and DFT calculations, that an electron transfer is active from the oxide to the aromatic molecules [7], opposed to that proposed by Dellinger. Accordingly the trend of the oxides in promoting the EPFR formation is parallel to their ability to stabilize the superoxide anion.
By collecting all these suggestions and mainly following the Cosentino mechanism [5], it seems to me that like in the case of CuO, a trigger action was played by O2 also for ZnO. It is well known that the superoxide species Zn2+-O2− stabilizes at the oxide surface [8] Thus Zn2+-O2− can be thought the product of the electron transfer from ZnO to O2 (probably via conduction electrons) and should allow the formation of the phenoxy radical on ZnO as well as on CuO, without reduction of Zn2+(A4 and A5 in Scheme 1b).
A similar mechanism was reported by Orlandi, Morazzoni et al. [9] and is concerning the catalytic formation of the phenoxy radical by interaction between E-methyl ferulate (ROH) and [N,N’bis(salicylidene)ethane-1,2-diaminato] cobalt(II), [Co(salen)]. The ESR investigation demonstrated the following pathway.
where the O2 interaction allows the formation of the superoxide derivative [Co(salen)(ROH)(O2−)] (step 1). The coordination of a second E-methyl ferulate, (step 2), removes O2− and gives rise to the phenoxy radical derivative. The above paper [9] also details that in the presence of a stronger electron donor molecule (like methanol of pyridine), the superoxide derivative remains stable and the phenoxy radical does not form.
It seems to us that the role of Zn 2+ can be thought as parallel to that of Cobalt(II) in the organometallic process, that is the formation of the phenoxy radical on ZnO goes through that of a superoxide species. If this has high stability, the formation of the phenoxy radical is hindered. In agreement with this opinion, the recent results reported by D’Arienzo et al. [10] outline that the phenoxy radical becomes stable on ZnO when the superoxide anion does not form. If, like in the case of defective ZnO, reactive oxygen vacancies are present and stabilize Zn2+-O2−, the formation of the phenoxy radical is strongly inhibited.
The way of avoiding the formation of phenoxy radicals is to not diffuse oxide particles with regular surfaces.
References
Lomnicki S, Truong H, Vejerano E, Dellinger B (2008) Copper oxide—based model of persistent free radical formation on combustion-derived particulate matter. Environ Sci Technol 42:4982–4988
Kelley M, Hebert VH, Thibeaux TM, Orchard MA, Hasan F, Cormier SA, Thevenot TP, Lomnicki SM, Varner KJ, Dellinger B (2013) Model combustion–generated particulate matter containing persistent free radicals redox cycle to produce reactive oxygen species. Chem Res Toxicol 26:1862–1871
Khachatryan L, Vejerano E, Lomnicki S, Dellinger B (2011) Environmentally persistent free radicals (EPFRs). 1. generation of reactive oxygen species in solution. Environ Sci Technol 45:8559–8566
Truong H, Lomnicki S, Dellinger B (2010) Potential for misidentification of environmentally persistent free radicals as molecular pollutants. Environ Sci Technol 44:1933–1939
D’Arienzo M, Gamba L, Morazzoni F, Cosentino U, Greco C, Lasagni M, Pitea D, Moro G, Cepek C, Butera V, Sicilia E, Russo N, Munoz-Garcia AB, Pavone M (2018) Experimental and theoretical investigation on the catalytic generation of environmentally persistent free radical from benzene. J Phys Chem C 121:9381–9393
Yang L, Liu G, Zheng M, Jin R, Zhao Y, Wu X, Xu Y (2017) Pivotal role of metal oxides in the formation of environmentally persistent free radicals. Environ Sci Technol 51:12329–12336
Petterson MC, DiTusa MF, McFerrin CA, Kurtz RI, Hall RW, Poliakoff ED, Sprunger PT (2017) Formation of environmentally persistent freer radicals (EPFRs) on ZnO at room temperature: implications for the fundamental model of EPFR generation. Chem Phys Lett 670:5–10
Morazzoni F, Scotti R, Minnaja N (1991) Reactivity of the surface paramagnetic species on polycrystalline ZnO and ZnO-supported ruthenium with hydrogen and carbon monoxide: an electron paramagnetic resonance invstigation. J Chem Soc Faraday Trans 87:493–496
Bolzacchini E, Canevali C, Morazzoni F, Orlandi M, Rindone B (1997) Spectromagnetic investigation of the active species in the oxidation of propenoidic phenols catalysed by [N, N’-bis(salycilidene)-ethane-1,2 diam inato]cobalt(II). J Chem Soc Dalton Trans. https://doi.org/10.1039/a705188c
D’Arienzo M, Mostoni S, Crapanzaro R, Cepek C, Di Credico B, Fasoli M, Polizzi S, Vedda A, Villa I, Scotti R (2019) Insight into the influence of ZnO defectivity on the catalytic generation of environmentally persistent free radicals in ZnO/SiO2 systems. J Phys Chem C 123:21651–21661
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflict of interest for the present paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morazzoni, F. Reexamining the Formation of Environmentally Persistent Free Radicals from Aromatic Molecules on Metal Oxides. Catal Lett 152, 2235–2238 (2022). https://doi.org/10.1007/s10562-021-03830-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03830-2