Abstract
We investigated the effect of supports based on ZrO2, TiO2, Al2O3, and SiO2 on the rate of propene formation in the metathesis of ethylene with 2-butene at 50 °C over Mo-containing catalysts possessing highly dispersed MoOx. Large improvements in this rate were achieved when using supports composed of mixed oxides (ZrO2–SiO2, ZrO2–PO4, TiO2–SiO2; Al2O3–SiO2) rather than of individual oxides (ZrO2, TiO2, Al2O3, SiO2). Although previous literature studies dealing with the metathesis reaction over Al2O3- or SiO2-suppported catalysts at higher temperatures suggest the importance of redox or acidic properties of supported MoOx species for catalyst activity, we were not able to establish any general direct correlation in this regard. Contrarily, the rate of propene formation can be significantly enhanced when promoting supports with an oxide promoter. We suggest that the created support lattice defects may facilitate the transformation of MoOx to Mo carbenes under reaction conditions or improve the intrinsic activity of the latter.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Metathesis of ethylene with 2-butene is one of the industrial processes for on-purpose production of propene [1]. Typical heterogeneous catalysts include oxides of Re, W, or Mo supported on SiO2, Al2O3, or SiO2–Al2O3 [2]. WOx/SiO2 applied on the large scale is characterized by low price, long lifetime, and resistance to poisoning [3,4,5]. WOx-based catalysts are, however, active at relatively high temperatures (∼350–450 °C), while MoOx- or ReOx-based catalysts demonstrate high activity at lower temperatures (25–200 °C) [2, 6].
The activity of MoOx-containing catalysts depends on polymerization degree of MoOx species, surface acidity, pretreatment conditions or presence of co-catalyst [7,8,9]. Isolated di-oxo or oligomeric mono-oxo supported MoOx show high activity for propene production, while crystalline MoO3 is not active [10, 11]. The influence of surface acidity on the activity of Mo-based catalysts was investigated in several independent works [9, 12,13,14]. Apparently, Brønsted acidic sites in close vicinity to MoOx species are relevant for high propene production at temperatures above 100 °C. The activity can also be enhanced after thermal catalyst pretreatment in inert gas [15], propene, ethylene, methane [16], methanol [13] or after photoreductive treatment in CO or H2. [17] The influence of co-catalysts such as CaO, Al2O3 or SiO2–Al2O3 on the metathesis of ethylene and 2-butene to propene over MoOx/AlxSiyOz was investigated by Goelden et al. [8]. Although these co-catalysts are inactive in this reaction, the activity of Mo-based catalysts is improved when one of them is used as an upstream-located pre-bed for the main catalyst.
To the best of our knowledge, most of previous studies of the metathesis of ethylene with 2-butene to propene were carried out with catalysts based on SiO2, Al2O3, or SiO2–Al2O3 supports. Thus, we decided to check the potential application of catalysts based on TiO2- or ZrO2-containing supports. From a fundamental viewpoint, it was important to check if the general activity-governing relationships established for the often-tested catalysts are also relevant for the-above-mentioned alternatives and particularly at 50 °C. To this end, we prepared a series of Mo-containing catalysts possessing highly dispersed MoOx species on the surface of TiO2- or ZrO2-based supports as well as of SiO2, Al2O3 and SiO2–Al2O3. They were tested for their activity in the metathesis of ethylene with 2-butene and characterized by complementary state-of-the-art techniques.
2 Experimental Part
2.1 Catalyst Synthesis
Several in-house prepared (SiO2, see Supporting Information (SI)) and commercial (Al2O3 (Chempur neutral), ZrO2–SiO2 (~ 5 wt% SiO2, Saint Gobain NorPro), ZrO2–PO4 (8 wt% PO4, Daiichi Kigenso Kagaku-Kogyo Co), TiO2-SiO2 (Ti/Si ratio = 9/1, Sachtleben), ZrO2 (Daiichi Kigenso Kagaku-Kogyo Co), and TiO2 (P25, Degussa)) supports were used for catalyst preparation. The commercial supports were calcined at 500 °C for 8 h. The catalysts were prepared by an incipient wetness impregnation method. Each support was impregnated with an aqueous solution of (NH4)6Mo7O24·4H2O (Riedel de Haen) to obtain the nominal metal surface density of 1.5 nm−2. Hereafter, the catalyst precursors were dried at 110 °C overnight and calcined at 500 °C for 8 h (at 575 °C for SiO2-supported catalysts for 16 h). The obtained catalysts are abbreviated as 1.5Mo/X, with “X” standing for support. The amounts of metal precursor and water required for catalyst synthesis were calculated by considering specific surface areas of supports and their absorptive capacity (Table S1 in SI). 1.5Mo/Siral 10 (10 wt% SiO2 in Al2O3), 1.5Mo/Siral 40 (40 wt% SiO2 in Al2O3), and 1.5Mo/Siral 70 (70 wt% SiO2 in Al2O3) are from our previous study [18].
2.2 Catalyst Characterization
Specific surface area (SBET) of the calcined supports was determined by nitrogen physisorption experiments at − 196 °C using a Belsorp mini II setup (Bel Japan). Adsorption isotherms were evaluated according to the BET method using the adsorption data in the relative pressure (p/p0) range of 0.05–0.3.
UV–vis measurements were carried out using an Avantes spectrometer (AvaSpec-2048-USB2-RM) equipped with a high-temperature reflection UV–vis probe, an Ava-Light-DH-S-BAL deuterium-halogen light source and a CCD array detector. Each sample was in situ calcined in flowing air at 500 °C for 1 h and cooled down to 50 °C in the same flow. The UV–vis spectra were recorded at 50 °C in the range of 200–800 nm. BaSO4 was used as a white standard for calculating the Kubelka–Munk function.
TEM analysis was conducted on a FEI Tecnai G 2 20 S-TWIN instrument operating at 200 kV. Each sample was ground into a fine powder and dispersed in ethanol using an ultrasonic bath. Hereafter, it was transferred onto copper grids coated with a 2 nm carbon layer and dried at 60 °C for 5 min. Additionally, a second copper grid coated with 2 nm carbon layer was placed on top.
Temperature-programmed desorption of ammonia (NH3-TPD) was carried out in an in-house developed setup containing eight individually heated continuous-flow fixed-bed quartz reactors. Each sample (50 mg) was calcined in flowing air at 500 °C for 1 h, cooled down to 120 °C, and purged with Ar for 15 min. Hereafter, the treated materials were exposed to a flow of 1 vol% NH3 in Ar (10 mL·min−1) at 120 °C for 1 h, flushed with Ar for 5 h to remove weakly bound NH3, and cooled down to 80 °C in the same flow. Then, they were heated in Ar flow up to 900 °C with a heating rate of 10 K·min−1. Desorbed ammonia was registered by an on-line mass spectrometer (Pfeiffer Vacuum OmniStar GSD 320). The signals at m/z of 15 (NH) and 40 (Ar) were recorded, with the latter being a reference standard.
For distinguishing between Brønsted and Lewis acidic sites, we performed IR measurements of adsorbed pyridine on a Tensor 27 spectrometer (Bruker). Prior to the measurement, each catalyst was pressed into a self-supporting wafer with a diameter of 20 mm. Prior to pyridine adsorption, the wafer was calcined in air flow at 400 °C in the IR cell for 10 min and cooled down in N2 flow to room temperature. Pyridine was adsorbed at 25 °C until saturation. Then the reaction cell was evacuated for removing physisorbed pyridine. After heating the sample in vacuum up to 150 °C, the IR spectrum of adsorbed pyridine was recorded.
Temperature-programmed reduction with H2 (H2-TPR) was carried out in the same set-up used for NH3-TPD. 100 mg of each sample were heated in flowing air to 500 °C for 1 h, cooled down to room temperature and purged with Ar for 15 min. Hereafter, the catalysts were heated in a flow of 5 vol% H2 in Ar (10 mL·min−1) up to 900 °C with a heating rate of 10 K·min−1. MS signals at m/z of 2 (H2) and 40 (Ar) were recorded.
Temperature-programmed surface reaction with trans-2-C4H8 (C4H8-TPSR) was carried out in the same set-up used for NH3-TPD and H2-TPR. 100 mg of each sample were heated in flowing air up to 500 °C for 1 h and then cooled down in Ar flow to room temperature. Hereafter, the catalysts were heated in a flow of 5 vol% C4H8 in Ar (10 mL·min−1) up to 700 °C with a heating rate of 10 K·min−1. The signals at m/z of 56 (C4H8), 43(CH3CO), 42 (C3H6), 44 (CO2), 18 (H2O), and 40 (Ar) were recorded.
In situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) measurements were collected using a Thermo Scientific Nicolet iS10 spectrometer equipped with a Harrick Praying Mantis and high-temperature reaction chamber. Prior to collecting the spectra, each sample was heated in N2 flow (10 mL·min−1, heating rate of 10 K·min−1) from room temperature to 450 °C, then treated in air flow (12 mL min−1) at 450 °C for 1 h and cooled down in N2 flow (10 mL min−1) to 150 °C. The spectra were collected with a resolution of 4 cm−1 and an accumulation of 64 scans in the range of 400–4000 cm−1 under flowing N2 (10 mL·min−1).
2.3 Catalytic Tests
Catalytic tests were performed at 1.25 bar (abs.) in an in-house developed set-up equipped with 14 continuous-flow fixed-bed quartz reactors. The catalysts (20 mg for 1.5Mo/Siral 10, 1.5Mo/Siral 40, and 1.5Mo/Siral 70 or 100 mg for the remaining catalysts, sieve faction of 315–710 µm) were heated in flowing N2 up to 500 °C and calcined in air flow at 500 °C for 3 h. Hereafter, they were cooled down in N2 flow to 50 °C and exposed to a flow (22 mL·min−1 per reactor) of C2H4/trans-2-C4H8/N2 = 5/5/1. All gases were purified over molecular sieve 3 Å filters (Roth). Nitrogen was further purified with an additional AlO-750-2 filter (Pure Gas Products).
The feed components and the reaction products (propene, cis-2-butene and traces of C5-olefins) were analysed by an on-line gas chromatograph (Agilent 6890) equipped with AL/S capillary column (for hydrocarbons), connected to a flame ionization detector and a PLOT/Q (for CO2)/Molsieve 5 (for H2, O2, N2, and CO) capillary column combination connected to a thermal conductivity detector. To calculate the initial rate of propene formation related to one Mo atom, i.e. turnover frequency of propene formation (TOFMo) Eq. 1 was used. The rate was determined after 420 s on stream at a degree of ethylene conversion below 15%. No catalyst activation but continuous deactivation was observed during 35 min on stream.
Here, \({F}_{feed}\) is the volumetric flow rate of the feed gas (mL·min−1) under reference conditions (0 °C, 1 atm), \({x}_{{N}_{2}}\) with superscripts “in” or “out” stand for the molar flow (mol·mL−1) of N2 at the reactor inlet or outlet, \({x}^{out}({C}_{3}{H}_{6})\) is the molar fraction of propene at the reactor outlet, \({N}_{A}\) is the Avogadro’s number (6.02 × 1023mol−1), \({V}_{m}\) is the molar volume (22,414 mL·mol−1), and \({m}_{cat}\) is catalyst weight (g), \(1.5\times {10}^{18}\) stands for the apparent surface density of Mo (1.5 × 1018 m−2), \({S}_{BET}\) is the specific surface area (m2 g−1).
3 Results and Discussion
3.1 Supported MoOx Species and Their Formation
The catalysts were treated at 500°C in air before recording the UV-vis spectra at 50°C in N2 atmosphere. The UV–vis spectra of the catalysts (Fig. 1) are characterized by strong absorption bands at 250 nm and 295 nm, related to highly dispersed and small linear-chained tetrahedral MoOx species. A band at ~ 330 nm can be assigned to octahedral polymerized MoOx species [9]. As no absorption band at 400 nm ascribed to small MoO3 crystallites [19] could be identified, all the catalysts should possess highly dispersed MoOx as a result of low surface density of Mo (1.5 nm−2). Strong absorption background in the range 200–400 nm for the TiO2-containing samples is due to the intrinsic bandgap of TiO2 (~ 3.16 eV) [20].
To prove additionally high dispersion of MoOx species, selected catalysts (1.5Mo/ZrO2, 1.5Mo/ZrO2-PO4, 1.5Mo/Siral 40) were characterized by transmission electron microscopy (TEM). Some representative TEM images of the catalysts are shown in Figure S1. Since no evidence of the existence of MoOx nanoparticles can be observed, MoOx species seem to be highly dispersed in agreement with the results of our UV–vis analysis (Fig. 1).
We also applied in situ DRIFTS to analyze if and how surface hydroxyl sites of support materials participate in the formation of supported MoOx species. The DRIFT spectra of dehydrated supports and the corresponding catalysts in the hydroxyl vibration region are shown in Figure S2. ZrO2 support possesses terminal Zr–OH (~ 3763 cm−1), bi-bridged Zr2–OH (~ 3729 cm−1), and tribridged Zr3–OH (~ 3678 cm−1 and ~ 3669 cm−1) species [21]. Mixed ZrO2–SiO2 is characterized by the presence of terminal Zr–OH (~ 3764 cm−1), terminal Si–OH (~ 3738 cm−1) as well as multi-coordinated hydroxyls absorbing at similar wavenumbers as those observed for ZrO2 (~ 3725 cm−1, ~ 3678 cm−1, and ~ 3665 cm−1). Terminal Zr–OH (~ 3762 cm−1) and at least two types of multicoordinated hydroxyls (~ 3671 cm−1, ~ 3640 cm−1) exist on the surface of ZrO2–PO4. TiO2 (P25) possesses several types of surface sites classified by Deiana et al. [22] as linear OH (ν(OH) > 3680 cm−1) and bridged (ν(OH) < 3680 cm−1). Each type of hydroxyl species has more than one local structure since several vibrations were observed (3717 cm−1, 3686 cm−1, 3668 cm−1, 3640 cm−1). Mixed TiO2-SiO2 possesses terminal Si–OH (~ 3737 cm−1), terminal Ti–OH (~ 3720 cm−1, ~ 3700 cm−1) and at least three types of multi-coordinated hydroxyls (~ 3677 cm−1, ~ 3662 cm−1, ~ 3639 cm−1). The DRIFT spectrum of Al2O3 support has at least 5 different bands related to hydroxyl species differing in the coordination number of both OH groups and Al cations. Acid strength of OH groups increases with decreasing the corresponding wavenumber of the absorption band [23]. The observed bands can be related to the terminal Al–OH (AlO3–OH at 3788 cm−1 and AlO5–OH at 3748 cm−1), bridged Al–OH–Al (AlO5–OH–AlO5 at 3723 cm−1 and AlO5–OH–AlO3 at 3698 cm−1), and tricoordinated (AlO5)3–OH at 3679 cm−1.[24] The DRIFT spectrum of SiO2 is characterized by an absorption band at around 3743 cm−1 related to isolated Si–OH (silanols) [25]. The DRIFT spectra of mixed Al2O3-SiO2 oxides (Siral 10, Siral 40, Siral 70) are characterized by the presence of bands at wavenumbers similar to those for SiO2 (~ 3736–3740 cm−1) and Al2O3 (3788 cm−1, 3723 cm−1, 3698 cm−1, 3679 cm−1) implying the existence of several types of hydroxyls including terminal Si–OH and Al–OH as well as multi-coordinated ones. It is however worth mentioning that the highly intensive band related to Si–OH sites overlaps with those related to OH sites bonded to Al cations. Moreover, increasing SiO2 content in mixed Al2O3–SiO2 samples leads to the appearance of geminal Si–OH**Si–OH hydroxyls characterized by a poorly resolved broad band at around 3600 cm−1 that complicates observation of other possible bands in this region.
Deposition of MoOx species on support material resulted in consumption (partial or complete) of some hydroxyls, which are, therefore, can be considered as anchoring sites. For example, terminal Zr–OH present on the surface of ZrO2 (band at 3763 cm−1) and ZrO2–SiO2 (band at 3764 cm−1) should actively participate in the formation of MoOx species. Similarly, terminal Ti–OH groups in TiO2 are also involved in this process. For other catalysts, no preferential consumption of certain OH groups for binding MoOx could be established.
3.2 Acidic and Redox Properties
In order to distinguish between Brønsted and Lewis acidic sites on the surface of catalysts, we performed IR measurements of adsorbed pyridine on all catalysts with the exception of those based on SiO2–Al2O3 supports (acidic properties of these catalysts were fully investigated in Ref. [9]). The obtained IR spectra are shown in Figure S3 in ESI. Noticeably, the spectra of all catalysts are characterized by the presence of bands typical for pyridine adsorbed on Lewis acidic sites (1600 and 1450 cm−1). The only catalyst also possessing Brønsted acidic sites (band at 1540 cm−1 in IR spectrum) is 1.5Mo/SiO2. Comparing the integral intensity of bands characteristic for different kind of acidic sites, it can be assumed that the number of Brønsted acidic sites in 1.5Mo/SiO2 is much lower than that of Lewis acidic sites. As the catalysts mainly contain Lewis acidic sites, it is possible to use NH3-TPD for deeper analysis of their acidic properties.
Figure 2a shows the NH3-TPD profiles of the catalysts and the corresponding support materials, while the temperature of the maximal rate of NH3 desorption and the number of acidic sites related to 1 nm2 of support are listed in Table S2. Among the support materials, TiO2 is characterized by the highest density of acidic sites (Nsup(a.s.)), while SiO2 does not possess acidic sites. Noticeably, the bare supports composed of a single oxide (except SiO2) have stronger acidic sites (high Tmax-NH3_sup) than their promoted/mixed counterparts. The maximum of NH3 desorption peak shifts to higher temperatures for most of the Mo-containing samples. Deposition of MoOx on ZrO2, Siral 10, Siral 40, Siral 70 and SiO2 leads to an increase in the total amount of acidic sites, while the presence of MoOx on ZrO2–SiO2, ZrO2–PO4, TiO2, TiO2–SiO2, and Al2O3 results in a decrease in the total number of acidic sites. Noticeably, for ZrO2–SiO2, ZrO2–PO4, TiO2–SiO2, and Al2O3, weak acidic sites are preferentially consumed upon deposition of MoOx species, while for other supports no preference can be observed.
Redox properties of the catalysts were analyzed by H2-TPR to check if and how the kind of support affects reducibility of supported MoOx species. For most catalysts, the H2-TPR profiles are characterized by several minima in the outlet H2 flow (Fig. 2b). They are due to H2 consumption through reaction with differently reducible MoOx species and/or bare support (at temperatures higher than 600 °C). The values of the temperature (Tmax-H2) of the first hydrogen consumption peak are shown in Table S2. With respect to Tmax-H2, MoOx supported on individual oxides can be ordered as follows: 1.5Mo/TiO2 < 1.5Mo/ZrO2 < 1.5Mo/Al2O3 < 1.5Mo/SiO2. In most cases, when the support is a promoted/mixed oxide, Tmax-H2 shifts to higher temperatures, i.e. reducibility of MoOx is worsened.
To study reducibility of MoOx species on different supports by trans-2-butene, temperature-programmed surface reaction (TPSR) tests with this olefin were also carried out. Oxygenates CH3COR, C3H6, CO2 and H2O were observed as reaction products. Among all catalysts, only 1.5Mo/ZrO2–PO4, 1.5Mo/TiO2–SiO2, 1.5Mo/Siral 10, 1.5Mo/Siral 40, and 1.5Mo/Siral 70 consumed C4H8 at relatively low temperature (Figure S4a). Such consumption was accompanied by formation of oxygenates and propene (Figure S4 (b) and (c) respectively). It is generally assumed that oxygenates are formed upon transformation of MoOx to carbenes through reaction with fed olefins [7, 10]. Accordingly, the formation of oxygenates in the present C4H8-TPSR tests can be considered as a proof of reductive activation of MoOx species. Propene formation can be explained through 1-and 2-butene metathesis. Thus, it can be concluded that activation of MoOx species supported on binary oxides (ZrO2–PO4, TiO2–SiO2, Al2O3–SiO2) proceeds easier than that of those supported on individual oxides (ZrO2, TiO2, Al2O3, SiO2) since no oxygenates and propene were formed at low temperature in the C4H8-TPSR tests with the latter materials. High-temperature C4H8 consumption (T > 400 °C) was observed for all catalysts and is related to oxidation of this olefin by MoOx to CO2 (Figure S4 (d)) and H2O (Figure S4 (e)).
3.3 Catalyst Activity in Metathesis of Ethylene with 2-Butene
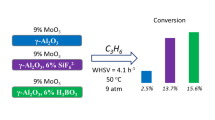
The rate of propene formation in the metathesis of ethylene with 2-butene was determined at 50 °C and related to one Mo atom to obtain an apparent turnover frequency (TOFMo). The calculated values are shown in Fig. 3. The catalysts based on individual metal oxides can be ordered in this regard as follows: 1.5Mo/Al2O3 > 1.5Mo/SiO2 > 1.5Mo/TiO2 > 1.5Mo/ZrO2. Importantly, the activity strongly increases when MoOx species are located on the surface of promoted/mixed oxides. Thus, the TOFMo values for 1.5Mo/ZrO2–PO4 and 1.5Mo/TiO2–SiO2 are more than 350 and 100 times higher than those for 1.5Mo/ZrO2 and 1.5Mo/TiO2, respectively. It should be noted that MoOx species supported on promoted ZrO2 or TiO2 reveal higher activity than those supported on bare Al2O3 or SiO2. Our catalysts are, however, less active than the most active state-of-the-art Mo-based catalysts based on SiO2–Al2O3 supports (Table S3) [13, 18, 26, 27].
The Mo-related rate of propene formation (TOFMo) determined at 50 °C over catalysts based on different supports. Ethylene conversion was below 1% over 1.5Mo/ZrO2, 1.5Mo/TiO2, 1.5Mo/Al2O3, and 1.5Mo/SiO2, about 2% over 1.5Mo/ZrO2–SiO2, 1.5Mo/ZrO2–PO4, and 1.5Mo/TiO2–SiO2, about 4% over 1.5/Siral 10 and 1.5/Siral 70 and about 11% over 1.5Mo/Siral 40
According to the results shown in Fig. 3, there is an obvious impact of the kind of support on catalyst activity. Considering the UV–vis spectra (Fig. 1), TEM (Figure S1), H2-TPR (Fig. 2b) results, and the Mo content well below a monolayer coverage, we are quite sure that our catalysts do not possess inactive MoO3, which would negatively affect the TOF values. However, we cannot exclude that Mo-carbenes formed from differently structured highly dispersed and small linear-chained tetrahedral MoOx species may differ in their intrinsic metathesis activity.
3.4 Factors Affecting Metathesis Activity of MoOx Species
Due to technical limitations for identification/quantification of Mo carbenes, we decided to follow a common approach used for metathesis catalysts tested at high temperatures to reveal possible origin(s) of different activity [9]. To this end we tried to correlate redox and acidic catalyst properties with catalyst activity. Redox properties of MoOx might play a crucial role due to the following reason. It is generally accepted that Mo = CHR carbenes are active sites for olefin metathesis [7, 13, 28, 29]. Such sites are assumed to be created through in situ activation of Mo6+Ox by olefin molecule into Mo4+Ox−1 followed by oxidative addition of another olefin molecule (Fig. 4).
Since the proposed pathway includes the reduction stage, it can be suggested that high reducibility of Mo6+Ox might be favorable for high activity. To check this idea, we tried to correlate the TOFMo values with the Tmax-H2 values given in Table S2 as well as with the temperature of C3H6 release peak observed in C4H8-TPSR profiles in Figure S4 (c). As no obvious relationships could be established (Fig. 5a and Figure S5), this catalyst property does not seem to be decisive.
The role of catalyst acidity in olefin metathesis is documented in several works [9, 12, 30]. Density functional theory calculations of the metathesis of ethylene with 2-butene over Mo/HBeta suggest that the presence of acidic sites could increase propene productivity due to decreasing the activation barrier for MoOx conversion into Mo-carbenes [12]. Based on the results of IR analysis of adsorbed pyridine, Hahn et al. [9] established a correlation between the concentration of Brønsted acidic sites in MoOx/Sirals and TOFMo determined at 150 °C, i.e. 100 °C higher than in the present study. Moreover, those authors considered the total amount of Brønsted acidic sites and did not pay attention to their strength. It can be however expected that the stronger the acidic sites, the higher the contribution of side reactions is as suggested in Ref. [12] To check if the strength of acidic sites and/or their concentration in the catalysts or support materials are important for propene formation at 50 °C over the present catalysts, we plotted the activity and the acidity data in Fig. 5b–d. Noticeably, most of the catalysts desorbing NH3 at lower temperatures are more active than those desorbing NH3 at higher temperatures (Fig. 5b). Accordingly, it can be assumed that weak acidic sites are preferable for metathesis reaction. Nevertheless, no general direct correlation between the activity and the strength of acidic sites could be established. Regarding the influence of concentration of acidic sites in the catalysts (Fig. 5c) or in the support materials (Fig. 5d) on the TOFMo values, no obvious dependence could also be found.
Accordingly, the direct influence of redox and acidic properties of the catalysts on the intrinsic activity of supported MoOx species in propene formation at 50 °C was not confirmed. It can be assumed that besides redox and acidic properties, the kind of support also influences the molecular structure of highly dispersed and small linear-chained tetrahedral MoOx species and therefore their ability to form carbenes and/or the intrinsic activity of the latter. Therefore, considering the molecular level complexity of the mechanism of carbene formation and the metathesis reaction itself, it is unlikely to establish general factors determining activity of MoOx species supported on different materials. Thus, further studies are required to check if there are universal fundamentals relevant for this reaction. In particularly, the role of support defects created upon promoting of supports with an oxide promoter seems to deserve a special attention.
4 Conclusions
For the first time, we have demonstrated that Mo-containing catalysts based on doped ZrO2 or TiO2 supports are attractive candidates for the metathesis of ethylene with 2-butenes to propene at only 50 °C and outperform their often-investigated counterparts based on individual SiO2 or Al2O3 supports. Their higher activity might be related to the presence of lattice defects created in support materials after promotion with an oxidic promoter. This knowledge opens a possibility for purposeful preparation of supports and, accordingly, catalysts on their basis for low-temperature propene formation from ethylene and 2-butenes.
References
Mol JC (2004) Industrial applications of olefin metathesis. J Mol Catal A 213(1):39–45
Lwin S, Wachs IE (2014) Olefin metathesis by supported metal oxide catalysts. ACS Catal 4(8):2505–2520
Suttibut P, Praserthdam P, Panpranot J (2017) Metathesis of ethylene and trans-2-butene over MgO admixed WO3/SiO2 catalysts. Eng J 21:1–16
Zuo G, Xu Y, Zheng J, Jiang F, Liu X (2018) Investigation on converting 1-butene and ethylene into propene via metathesis reaction over W-based catalysts. RSC Adv 8(15):8372–8384
Gayapan K, Sripinun S, Panpranot J, Praserthdam P, Assabumrungrat S (2018) Effect of pretreatment atmosphere of WOx/SiO2 catalysts on metathesis of ethylene and 2-butene to propylene. RSC Adv 8(21):11693–11704
Merle N, Le Quéméner F, Bouhoute Y, Szeto KC, De Mallmann A, Barman S et al (2017) Well-defined molybdenum oxo alkyl complex supported on silica by surface organometallic chemistry: a highly active olefin metathesis precatalyst. J Am Chem Soc 139(6):2144–2147
Kondratenko VA, Hahn T, Bentrup U, Linke D, Kondratenko EV (2018) Metathesis of ethylene and 2-butene over MoOx/Al2O3-SiO2: effect of MoOx structure on formation of active sites and propene selectivity. J Catal 360:135–144
Goelden V, Linke D, Kondratenko EV (2015) Investigation of the enhancing effect of solid cocatalysts on propene formation in ethene/trans-2-butene metathesis over MoOx/SiO2–Al2O3. ACS Catal 5(12):7437–7445
Hahn T, Bentrup U, Armbrüster M, Kondratenko EV, Linke D (2014) The enhancing effect of brønsted acidity of supported MoOx species on their activity and selectivity in ethylene/trans-2-butene metathesis. ChemCatChem 6(6):1664–1672
Chakrabarti A, Wachs IE (2018) Molecular structure-reactivity relationships for olefin metathesis by Al2O3-supported surface MoOx sites. ACS Catal 8(2):949–959
Debecker DP, Schimmoeller B, Stoyanova M, Poleunis C, Bertrand P, Rodemerck U et al (2011) Flame-made MoO3/SiO2-Al2O3 metathesis catalysts with highly dispersed and highly active molybdate species. J Catal 277(2):154–163
Li X, Zheng A, Guan J, Han X, Zhang W, Bao X (2010) The effect of support acidity on olefin metathesis over heterogeneous Mo/HBeta catalyst: a DFT study. Catal Lett 138(1):116–123
Amakawa K, Kröhnert J, Wrabetz S, Frank B, Hemmann F, Jäger C et al (2015) Active sites in olefin metathesis over supported molybdena catalysts. ChemCatChem 7(24):4059–4065
Consoli DF, Zhang S, Shaikh S, Román-Leshkov Y (2018) Influence of framework heteroatoms on olefin metathesis activity using MoO3-MFI catalysts. Org Process Res Dev 22(12):1683–1686
Grünert W, Stakheev AY, Feldhaus R, Anders K, Shpiro ES, Minachev KM (1992) Reduction and metathesis activity of MoO3/Al2O3 catalysts II. The activation of MoO3/Al2O3 catalysts. J Catal 135(1):287–299
Michorczyk P, Węgrzyniak A, Węgrzynowicz A, Handzlik J (2019) Simple and efficient way of molybdenum oxide-based catalyst activation for olefins metathesis by methane pretreatment. ACS Catal 9(12):11461–11467
Shelimov BN, Elev LV, Kazansky VB (1986) Use of photoreduction for activation of silica-molybdena catalysts for propylene metathesis: comparison with thermal reduction. J Catal 98(1):70–81
Otroshchenko T, Reinsdorf O, Linke D, Kondratenko EV (2019) A chemical titration method for quantification of carbenes in Mo- or W-containing catalysts for metathesis of ethylene with 2-butenes: verification and application potential. Catal Sci Technol 9(20):5660–5667
Lee EL, Wachs IE (2007) In situ spectroscopic investigation of the molecular and electronic structures of SiO2 supported surface metal oxides. J Phys Chem C 111(39):14410–14425
Shao J, Sheng W, Wang M, Li S, Chen J, Zhang Y et al (2017) In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl Catal B 209:311–319
Kouva S, Honkala K, Lefferts L, Kanervo J (2015) Review: monoclinic zirconia, its surface sites and their interaction with carbon monoxide. Catal Sci Tech 5(7):3473–3490
Deiana C, Fois E, Coluccia S, Martra G (2010) Surface structure of TiO2 P25 nanoparticles: infrared study of hydroxy groups on coordinative defect sites. J Phys Chem C 114(49):21531–21538
Zhang B, Wachs IE (2021) Identifying the catalytic active site for propylene metathesis by supported ReOx catalysts. ACS Catal 11(4):1962–1976
Liu X (2008) DRIFTS study of surface of γ-alumina and its dehydroxylation. J Phys Chem C 112(13):5066–5073
Amakawa K, Wrabetz S, Kröhnert J, Tzolova-Müller G, Schlögl R, Trunschke A (2012) In Situ generation of active sites in olefin metathesis. J Am Chem Soc 134(28):11462–11473
Debecker DP, Stoyanova M, Colbeau-Justin F, Rodemerck U, Boissière C, Gaigneaux EM et al (2012) One-pot aerosol route to MoO3-SiO2-Al2O3 catalysts with ordered super microporosity and high olefin metathesis activity. Angew Chem Int Ed 51(9):2129–2131
Bouchmella K, Mutin PH, Stoyanova M, Poleunis C, Eloy P, Rodemerck U et al (2013) Olefin metathesis with mesoporous rhenium-silicium-aluminum mixed oxides obtained via a one-step non-hydrolytic sol-gel route. J Catal 301:233–241
Cheng Z, Lo CS (2015) Propagation of olefin metathesis to propene on WO3 catalysts: a mechanistic and kinetic study. ACS Catal 5(1):59–72
Chakrabarti A, Wachs IE (2019) Activation mechanism and surface intermediates during olefin metathesis by supported MoOx/Al2O3 catalysts. J Phys Chem C 123(19):12367–12375
Zhao P, Ye L, Sun Z, Lo BTW, Woodcock H, Huang C et al (2018) Entrapped single tungstate site in zeolite for cooperative catalysis of olefin metathesis with brønsted acid site. J Am Chem Soc 140(21):6661–6667
Acknowledgements
Financial support by Deutsche Forschungsgemeinschaft (DFG, Project OT 586/1-1) is gratefully acknowledged. The authors thank to Ralph Kraehnert for providing TEM images.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otroshchenko, T., Zhang, Q. & Kondratenko, E.V. Room-Temperature Metathesis of Ethylene with 2-Butene to Propene Over MoOx-Based Catalysts: Mixed Oxides as Perspective Support Materials. Catal Lett 152, 2366–2374 (2022). https://doi.org/10.1007/s10562-021-03822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03822-2