Abstract
Engineered laccases represent an eco-friendlier and robust biocatalytic tool for the treatment of dye-harboring textile wastewater. This study investigates the immobilization of purified laccase from Pleurotus sapidus onto firm-quality spherical Ca-alginate beads by a cross-linking approach. Sodium alginate at an optimal concentration of 4% (w/v) furnished the highest immobilization efficiency (69%). EDX analysis confirmed the detection of copper in the laccase-incorporated alginate beads. The optimum pH for free laccase was 3.0, while the Ca-alginate-Lac showed the maximum enzyme activity (440.2 U/mL) at pH 5.0. In contrast to a free enzyme (40 °C), immobilized laccase performed best at an elevated temperature of 65 °C. The Km and Vmax values in the case of free and Ca-alginate immobilized enzymes were 114 µM, 370 U/mL, and 123 µM, 548 U/mL, respectively. Immobilized laccase catalyzed a highly efficient decolorization of various reactive and disperse dye pollutants and recorded in the range of 86.19–91.01%. The COD and TOC levels were substantially reduced to 91.90–94.94 and 77.01–93.29%, respectively, in the maximally degraded dye solutions. Therefore, immobilization of laccase on Ca-alginate beads offers a cost-effective and facile method for environmental remediation applications.
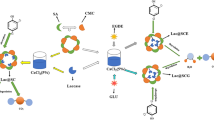
Graphic Abstract









Similar content being viewed by others
References
Bilal M, Rasheed T, Nabeel F, Iqbal HM, Zhao Y (2019) Hazardous contaminants in the environment and their laccase-assisted degradation–a review. J Environ Manag 234:253–264
Chauhan PS, Goradia B, Saxena A (2017) Bacterial laccase: recent update on production, properties and industrial applications. 3 Biotech 7(5):323
Kashefi S, Borghei SM, Mahmoodi NM (2019) Covalently immobilized laccase onto graphene oxide nanosheets: preparation, characterization, and biodegradation of azo dyes in colored wastewater. J Mol Liq 276:153–162
Kishor R, Purchase D, Saratale GD, Saratale RG, Ferreira LFR, Bilal M, Chandra R, Bharagava RN (2021) Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J Environ Chem Eng 9:105012
Muhammad Z, Ali F, Sajjad M, Ali N, Bilal M, Shaik MR, Adil SF, Sharaf MAF, Awwad EM, Khan M (2021) Zirconium-doped chromium IV oxide nanocomposites: synthesis, characterization, and photocatalysis towards the degradation of organic dyes. Catalysts 11(1):117
Singh AK, Bilal M, Iqbal HM, Raj A (2021) Trends in predictive biodegradation for sustainable mitigation of environmental pollutants: Recent progress and future outlook. Sci Total Environ 770:144561
Ali N, Bilal M, Khan A, Ali F, Yang Y, Malik S, Din SU, Iqbal HM (2021) Deployment of metal-organic frameworks as robust materials for sustainable catalysis and remediation of pollutants in environmental settings. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.129605
Catapane M, Nicolucci C, Menale C, Mita L, Rossi S, Mita DG, Diano N (2013) Enzymatic removal of estrogenic activity of nonylphenol and octylphenol aqueous solutions by immobilized laccase from Trametes versicolor. J Hazard Mater 248:337–346
Hayat H, Mahmood Q, Pervez A, Bhatti ZA, Baig SA (2015) Comparative decolorization of dyes in textile wastewater using biological and chemical treatment. Sep Purif Technol 154:149–153
Mehandia S, Sharma SC, Arya SK (2020) Immobilization of laccase on chitosan-clay composite beads to improve its catalytic efficiency to degrade industrial dyes. Mater Today Commun 25:101513
Srinivasan A, Viraraghavan T (2010) Decolorization of dye wastewaters by biosorbents: a review. J Environ Manag 91(10):1915–1929
Addorisio V, Sannino F, Mateo C, Guisan JM (2013) Oxidation of phenyl compounds using strongly stable immobilized-stabilized laccase from Trametes versicolor. Process Biochem 48(8):1174–1180
Asgher M, Noreen S, Bilal M (2017) Enhancement of catalytic, reusability, and long-term stability features of Trametes versicolor IBL-04 laccase immobilized on different polymers. Int J Biol Macromol 95:54–62
Chao C, Zhao Y, Guan H, Liu G, Hu Z, Zhang B (2017) Improved performance of immobilized laccase on poly (diallyldimethylammonium chloride) functionalized halloysite for 2, 4-dichlorophenol degradation. Environ Eng Sci 34(10):762–770
Lou C, Jing T, Zhou J, Tian J, Zheng Y, Wang C, Zhao Z, Lin J, Liu H, Zhao C, Guo Z (2020) Laccase immobilized polyaniline/magnetic graphene composite electrode for detecting hydroquinone. Int J Biol Macromol 149:1130–1138
Sondhi S, Kaur R, Kaur S, Kaur PS (2018) Immobilization of laccase-ABTS system for the development of a continuous flow packed bed bioreactor for decolorization of textile effluent. Int J Biol Macromol 117:1093–1100
Wang J, Yu S, Feng F, Lu L (2019) Simultaneous purification and immobilization of laccase on magnetic zeolitic imidazolate frameworks: recyclable biocatalysts with enhanced stability for dye decolorization. Biochem Eng J 150:107285
Ardhaoui M, Bhatt S, Zheng M, Dowling D, Jolivalt C, Khonsari FA (2013) Biosensor based on laccase immobilized on plasma polymerized allylamine/carbon electrode. Mater Sci Eng C 33(6):3197–3205
Li Y, Chen SM, Chen WC, Li YS, Ali MA, AlHemaid FMA (2011) Platinum nanoparticles (PtNPs)–laccase assisted biocathode reduction of oxygen for biofuel cells. Int J Electrochem Sci 6:6398–6409
Lloret L, Eibes G, Feijoo G, Moreira MT, Lema JM (2012) Continuous operation of a fluidized bed reactor for the removal of estrogens by immobilized laccase on Eupergit supports. J Biotechnol 162(4):404–406
Moldes D, Cadena EM, Vidal T (2010) Biobleaching of eucalypt kraft pulp with a two laccase-mediator stages sequence. Biores Technol 101(18):6924–6929
Witayakran S, Ragauskas AJ (2009) Modification of high-lignin softwood kraft pulp with laccase and amino acids. Enzyme Microb Technol 44(3):176–181
Tišma M, Šalić A, Planinić M, Zelić B, Potočnik M, Šelo G, Bucić-Kojić A (2020) Production, characterisation and immobilization of laccase for an efficient aniline-based dye decolourization. J Water Process Eng 36:101327
Wen X, Zeng Z, Du C, Huang D, Zeng G, Xiao R, Lai C, Xu P, Zhang C, Wan J, Hu L, Yin L, Zhou C, Deng R (2019) Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 222:865–871
Feng Y, Zhong L, Bilal M, Tan Z, Hou Y, Jia S, Cui J (2019) Enzymes@ ZIF-8 nanocomposites with protection nanocoating: stability and acid-resistant evaluation. Polymers 11(1):27
Ren S, Li C, Jiao X, Jia S, Jiang Y, Bilal M, Cui J (2019) Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem Eng J 373:1254–1278
Ren S, Wang Z, Bilal M, Feng Y, Jiang Y, Jia S, Cui J (2020) Co-immobilization multienzyme nanoreactor with co-factor regeneration for conversion of CO2. Int J Biol Macromol 155:110–118
Gasser CA, Ammann EM, Shahgaldian P, Corvini PFX (2014) Laccases to take on the challenge of emerging organic contaminants in wastewater. Appl Microbiol Biotechnol 98(24):9931–9952
Nair RR, Demarche P, Agathos SN (2013) Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. New Biotechnol 30(6):814–823
Rahmani K, Faramarzi MA, Mahvi AH, Gholami M, Esrafili A, Forootanfar H, Farzadkia M (2015) Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int Biodeterior Biodegrad 97:107–114
Reda FM, Hassan NS, El-Moghazy AN (2018) Decolorization of synthetic dyes by free and immobilized laccases from newly isolated strain Brevibacterium halotolerans N11 (KY883983). Biocatal Agric Biotechnol 15:138–145
Vakili M, Deng S, Liu D, Li T, Yu G (2019) Preparation of aminated cross-linked chitosan beads for efficient adsorption of hexavalent chromium. Int J Biol Macromol 139:352–360
Asgher M, Wahab A, Bilal M, Iqbal HM (2018) Delignification of lignocellulose biomasses by alginate–chitosan immobilized laccase produced from Trametes versicolor IBL-04. Waste Biomass Valoriz 9(11):2071–2079
Bilal M, Iqbal HM (2019) Lignin peroxidase immobilization on Ca-alginate beads and its dye degradation performance in a packed bed reactor system. Biocatal Agric Biotechnol 20:101205
Bilal M, Asgher M, Iqbal HM, Hu H, Zhang X (2017) Delignification and fruit juice clarification properties of alginate-chitosan-immobilized ligninolytic cocktail. LWT 80:348–354
Qamar SA, Asgher M, Bilal M (2020) Immobilization of alkaline protease from Bacillus brevis using Ca-alginate entrapment strategy for improved catalytic stability, silver recovery, and dehairing potentialities. Catal Lett 150:3572–3583
Daâssi D, Rodríguez-Couto S, Nasri M, Mechichi T (2014) Biodegradation of textile dyes by immobilized laccase from Coriolopsis gallica into Ca-alginate beads. Int Biodeterior Biodegrad 90:71–78
Aslam S, Asgher M, Hussain F, Khan NA (2016) Exploration of optimum operating conditions for enhanced laccase enzyme production by Pleurotus nebrodensis WC 850 through response surface methodology. J Animal Plant Sci 26(3):794–804
Gulay S, Sanl- Mohamed G (2012) Immobilization of thermoalkalophilic recombinant esterase enzyme by entrapment in silicate coated Ca-alginate beads and its hydrolytic properties. Int J Biol Macromol 50:545–551
Greenberg AE, Trussell RR, Clesceri LS (1985) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, Washington
Kansoh AL, M-Ali A (2001) Xylanolytic activities of Streptomyces sp. 1—taxonomy, production, partial purification and utilization of agricultural wastes. Acta Microbiol Immunol Hung 48(1):39–52
Mtui G, Nakamura Y (2008) Lignocellulosic enzymes from Flavodon flavus, a fungus isolated from Western Indian Ocean off the coast of Dar es Salaam, Tanzania. Afr J Biotechnol 7(17):3066
Hadri SH, Asad MJ, Gulfraz M, Asghar M, Minhas NM, Mahmood RT, Zakia S, Mahmood N (2015) Solid State Fermentation for the production of Laccase by Neurospora sitophila using agro-wastes and its partial purification. Int J Biochem Biotechnol 4(5):564–573
Aslam S, Asgher M (2011) Partial purification and characterization of ligninolytic enzymes produced by Pleurotus ostreatus during solid state fermentation. Afr J Biotech 10(77):17875–17883
Mtui G, Msh AM, Johansson G, Kivaisi A (2009) Purification and characterization of a laccase from the basidiomycete Funalia trogii (Berk.) isolated in Tanzania. Afr J Biochem Res 3(5):250–258
Bilal M, Asgher M, Shahid M, Bhatti HN (2016) Characteristic features and dye degrading capability of agar-agar gel immobilized manganese peroxidase. Int J Biol Macromol 86:1–37
Geethanjali S, Subash A (2013) Optimization and immobilization of purified Labeo rohita visceral protease by entrapment method. Enzyme Res. https://doi.org/10.1155/2013/874050
Bilal M, Asgher M (2015) Dye decolorization and detoxification potential of Ca-alginate beads immobilized manganese peroxidase. BMC Biotechnol 15(1):1–14
Bilal M, Asgher M (2015) Sandal reactive dyes decolorization and cytotoxicity reduction using manganese peroxidase immobilized onto polyvinyl alcohol-alginate beads. Chem Cent J 9(1):1–14
Riaz A, Qader SAU, Anwar A, Iqbal S (2009) Immobilization of a thermostable α-amylase on calcium alginate beads from Bacillus subtilis KIBGE-HAR. Aust J Basic Appl Sci 3(3):2883–2887
Yavaşer R, Karagözler AA (2021) Laccase immobilized polyacrylamide-alginate cryogel: a candidate for treatment of effluents. Process Biochem 101:137–146
Uygun M (2013) Preparation of laccase immobilized cryogels and usage for decolorization. J Chem 2013:1
Wang Z, Ren D, Yu H, Jiang S, Zhang S, Zhang X (2020) Study on improving the stability of adsorption-encapsulation immobilized Laccase@ ZIF-67. Biotechnol Rep 28:e00553
Wehaidy HR, Abdel-Naby MA, El-Hennawi HM, Youssef HF (2019) Nanoporous zeolite-X as a new carrier for laccase immobilization and its application in dyes decolorization. Biocatal Agric Biotechnol 19:101135
Andriani D, Sunwoo C, Ryu HW, Prasetya B, Park DH (2012) Immobilization of cellulase from newly isolated strain Bacillus subtilis TD6 using calcium alginate as a support material. Bioprocess Biosyst Eng 35(1–2):29–33
Jaiswal N, Pandey VP, Dwivedi UN (2016) Immobilization of papaya laccase in chitosan led to improved multipronged stability and dye discoloration. Int J Biol Macromol 86:288–295
Quiroga E, Illanes CO, Ochoa NA, Barberis S (2011) Performance improvement of araujiain, a cystein phytoprotease, by immobilization within calcium alginate beads. Process Biochem 46(4):1029–1034
Mazlan SZ, Hanifah SA (2017) Effects of temperature and pH on immobilized laccase activity in conjugated methacrylate-acrylate microspheres. Int J Polym Sci 2017:1
Zheng F, Cui BK, Wu XJ, Meng G, Liu HX, Si J (2016) Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int Biodeterior Biodegrad 110:69–78
Kumar VV, Sivanesan S, Cabana H (2014) Magnetic cross-linked laccase aggregates—bioremediation tool for decolorization of distinct classes of recalcitrant dyes. Sci Total Environ 487:830–839
Zhang Y, Zhang Y, Wang H, Yan B, Shen G, Yu R (2009) An enzyme immobilization platform for biosensor designs of direct electrochemistry using flower-like ZnO crystals and nano-sized gold particles. J Electroanal Chem 627(1–2):9–14
Asgher M, Iqbal HMN, Asad MJ (2012) Kinetic characterization of purified laccase produced from Trametes versicolor IBL-04 in solid state bioprocessing of corncobs. BioResources 7(1):1171–1188
Amin R, Khorshidi A, Shojaei AF, Rezaei S, Faramarzi MA (2018) Immobilization of laccase on modified Fe3O4@ SiO2@ Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int J Biol Macromol 114:106–113
Ulu A, Birhanli E, Boran F, Köytepe S, Yesilada O, Ateş B (2020) Laccase-conjugated thiolated chitosan-Fe3O4 hybrid composite for biocatalytic degradation of organic dyes. Int J Biol Macromol 150:871–884
Arica MK, Salih B, Celikbicak O, Bayramoglu G (2017) Immobilization of laccase on the fibrous polymer grafted film and studies of textile dyes degradation by MALDI-ToF-MS. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2017.09.023
Rani M, Shanker U, Chaurasia AK (2017) Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: degradation of alizarin red S dye. J Environ Chem Eng 5:1–33
Omeje KO, Nnolim NE, Ezema BO, Ozioko JN, Eze SO (2020) Synthetic dyes decolorization potential of agroindustrial waste-derived thermo-active laccase from Aspergillus species. Biocatal Agric Biotechnol 29:1–7
Bilal M, Iqbal M, Hu H, Zhang X (2016) Mutagenicity and cytotoxicity assessment of biodegraded textile effluent by Ca-alginate encapsulated manganese peroxidase. Biochem Eng J 109:153–161
Acknowledgements
The authors are grateful to the Higher Education Commission (HEC), Islamabad, Pakistan, to provide funds for the present investigated research work. The analytical facilities provided by High Tech Lab, University of Agriculture, Faisalabad are thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslam, S., Ali, A., Asgher, M. et al. Fabrication and Catalytic Characterization of Laccase-Loaded Calcium-Alginate Beads for Enhanced Degradation of Dye-Contaminated Aqueous Solutions. Catal Lett 152, 1729–1741 (2022). https://doi.org/10.1007/s10562-021-03765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03765-8