Abstract
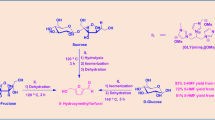
The present work was undertaken to ascertain whether IL-metal salt catalyzed isopropylidenation of d-fructose yield desired acetonides. For the first time, an imidazole based ionic liquid compound accompanied with strontium chloride has been identified as a suitable dual catalyst system for the chemoselective O-isopropylidenation of d-fructose with acetone. In the present protocol, mainly the kinetically controlled less stable cyclic ketal 1,2;4,5-di-O-isopropylidene-β-d-fructopyranose is formed as an initial product with satisfactory yield and without isomerization to the thermodynamically more stable cyclic ketal 2,3;4,5-di-O-isopropylidene-β-d-fructopyranose. Therefore, this protocol is more advantageous compared to other mineral acid catalyzed protocols that require more sensitive reaction conditions.
Graphic Abstract





Similar content being viewed by others
References
Pétursson S (1997) J Chem Educ 74:1297–1303
Kang J, Lim GJ, Yoon SK, Kim MY (1995) J Org Chem 60:564–577
Tu Y, Frohn M, Wang ZX, Shi Y (2003) Org Synth 80:1
Wang ZX, Tu Y, Frohn M, Zhang JR, Shi Y (1997) J Am Chem Soc 119:11224–11235
Costa PRR, Ferreira VF, Alencar KG, Filho HCA, Ferreira CM, Pinheiro S (1996) J Carbohydr Chem 15:691–699
Vanlaldinpuia K, Bora P, Bez G (2017) J Chem Sci 129:301–312
Huang H, Liu X, Chen S, Chen H, Zheng Z (2004) Tetrahedron: Asymmetry 15:2011–2019
Vennam RDK, Thatipamula RK, Haridasyam SB, Koppula SK (2018) Chem Heterocycl Comp 54:630–637
Veleti SK, Lindenberger JJ, Thanna S, Ronning DR, Sucheck SJ (2014) J Org Chem 79:9444–9450
Maryanoff BE, Nortey SO, Gardock JF, Shank RP, Dodgson SP (1987) J Med Chem 30:880–887
Beksan E, Schieberle P, Robert F, Blank I, Fay LB, Cerny HS, Hofmann T (2003) J Agric Food Chem 51:5428–5436
Dag A, Callari M, Lua H, Stenzel MH (2016) Polym Chem 7:1031–1036
Zhao J, Babiuch K, Lu H, Dag A, Gottschaldt M, Stenzel MH (2014) Chem Commun 50:15928–15931
Kiso M, Hasegawa A (1976) Carbohydr Res 52:95–101
Singh PP, Gharia MM, Dasgupta F, Srivastava HC (1977) Tetrahedron Lett 18:439–440
Kartha KPR (1986) Tetrahedron Lett 27:3415–3416
Rauter AR, Ribeiro FR, Fernandes AC, Figueiredo JA (1995) Tetrahedron 51:6529–6540
Asakura J, Matsubara Y, Yoshihara M (1996) J Carbohydr Chem 15:231–239
Manzo E, Barone G, Parrilli M (2000) Synlett 2000:887–889
Rajput VK, Mukhopadhyay B (2006) Tetrahedron Lett 47:5939–5941
Lin CC, Jan MD, Weng SS, Lin CC, Chen CT (2006) Carbohydr Res 341:1948–1953
Khan AT, Khan MM (2010) Carbohydr Res 345:154–159
Khan AT, Khan MM, Adhikary A (2011) Carbohydr Res 346:673–677
Mandal S, Verma PR, Mukhopadhyay B, Gupta P (2011) Carbohydr Res 346:2007–2010
Vanlaldinpuia K, Bez G (2011) Tetrahedron Lett 52:3759–3764
Rong YW, Zhang QH, Wang W, Li BL (2014) Bull Korean Chem Soc 35:2165–2168
Rokade SM, Bhate PM (2017) J Carbohydr Chem 36:20–30
Hough L, Richardson AC (1967) In: Coffey S (ed) Rodd’s Chemistry of Carbon Compounds, vol I-F. Elsevier, Amsterdam, p 356
Brady RF (1970) Carbohydr Res 15:35–40
Banks MR, Cadogan JIG, Gosney I, Gould RO, Hodgson PKG, McDougall D (1998) Tetrahedron 54:9765–9784
Meng XB, Li YF, Li Z (2007) J Carbohydr Res 342:1101–1104
Ohle H (1924) Ber Dtsch Chem Ges 57:1566–1576
Verhart CGJ, Caris BMG, Zwanenburg B, Chittenden GJF (1992) Recl Trav Chim Pays-Bas 111:348–352
Pedatella S, Guaragna A, D’Alonzo D, De Nisco M, Palumbo G (2006) Synthesis 2005:305–308
Yıldırım A, Mudaber S, Öztürk S (2019) Eur J Lipid Sci Technol 121:1800303
Polchow K, Voss J (2005) Phosphorus Sulfur Silicon Relat Elem 180:1755–1768
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yıldırım, A. An Expedient Method for Kinetically Controlled Acetonide Formation from d-Fructose Induced by Ionic Liquid Catalyst Accompanied with SrCl2·6H2O. Catal Lett 150, 2566–2571 (2020). https://doi.org/10.1007/s10562-020-03175-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03175-2