Abstract
A set of cerium–manganese–copper oxide catalysts with various foreign metal contents was prepared via the solution combustion synthesis (SCS). The catalysts were characterized by complementary techniques such as N2 physisorption at − 196 °C, X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), H2-temperature-programmed reduction (H2-TPR), O2-temperature-programmed desorption (O2-TPD) and X-ray photoelectron spectroscopy (XPS). Their catalytic activity was tested towards the VOC oxidation using ethylene and propylene as probe molecules. As a whole, it has been observed that the Ce55Mn45 sample (Mn 45 at.%), containing MnOx clusters interacting with the ceria phase, was the most active catalyst for propylene oxidation, exhibiting a complete conversion at 250 °C. On the other hand, the ternary oxide catalyst (Ce55Mn22.5Cu22.5 with Mn = 22.5 at.% and Cu = 22.5 at.%) has exhibited the best results for the oxidation of ethylene. These findings suggest that the co-presence of different active phases on the catalytic surface may have a beneficial (multiplicative) role on the whole reactivity. Finally, the most active powder catalysts were wash-coated in a SiC monolith and tested in a bench-scale reactor. As a whole, the catalyzed monoliths performed the complete oxidation of either ethylene or propylene at lower temperatures (550 and 450 °C, respectively) than those required to thermally decompose these molecules.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Volatile organic compounds (VOCs) include any organic substance having a boiling point equal or lower than 250 °C at standard pressure (i.e. 101.3 kPa). In EU as well as in several other countries in the world, there are already regulations and control of substances effectively classified as VOCs and their production at industrial level [1, 2]. These substances may derive from two different sources: biogenic, if they are produced in several processes occurring between the Earth’s crust and the atmosphere, or anthropogenic, if they result from human-related activities [3]. A virtually inevitable production of VOCs comes from household activities (e.g. cleaning, painting) or from various industrial activities where chemical and/or physical processes are performed, such as automotive, petrochemical, waste management and food processing [3]. When humans are exposed to VOCs, these compounds have demonstrated to represent a toxic, carcinogenic, mutagenic or teratogenic risk [4], which motivated further observations on airborne chemical contact in indoor environments [5, 6].
In this context, several abatement techniques have been developed to diminish the emissions of VOCs such as thermal or catalytic oxidation, adsorption, absorption and condensation [7]. Catalytic oxidation has demonstrated to be a suitable technique to perform an effective abatement of pollutants in gaseous streams containing variable flow rates and concentrations [8]. Some examples of the abated pollutants could be different hydrocarbons and diesel soot [9,10,11]. The latter can be oxidized through different mechanisms or kinetics depending on different factors (e.g. operating conditions, catalyst, reactants, etc.). The kinetic models that describe the VOCs oxidation over oxide catalysts typically involve the Langmuir–Hinshelwood and/or the Mars–van Krevelen-type mechanisms [9]. Different materials have shown promising performances in the catalytic oxidation of VOCs under various reaction conditions. Such materials can be supported noble metals (e.g. Pd, Pt, Ag and Au), which have shown to be active at low temperatures [9, 12]. Non-noble metal oxides like oxides of manganese, copper and cerium have also been widely studied. They are cheaper and more resistant to chemical poisoning than the noble metals as well as they can be active at relatively high temperatures [9, 12]. Mixed oxides have also shown to be active in the oxidation of several VOCs, due to possible synergies among the oxide phases in the catalyst [13,14,15,16].
Cerium (IV) oxide has demonstrated its efficacy in catalysis due to its redox properties and oxygen storage capacity, as well as it is able to endorse Mars–Van Krevelen (MvK)-type mechanisms [9, 16, 17]. Since the MvK mechanism occurs through oxygen species from the solid, the “redox-flexibility” of the material becomes a key factor for the catalytic oxidation reaction. Thus, both the electronic and structural changes caused by the introduction of foreign metals in the ceria lattice seem to be beneficial toward the VOC oxidation reactions.
It appears that doping leads to improvements in terms of structural stability, oxygen storage capacity and mobility. Moreover, the surface reducibility of ceria-based catalysts can be improved via the presence of two or more oxide phases (e.g. CeO2 with MnOx, CuOx) interacting with each other thus inducing the formation of oxygen vacancies [17,18,19,20,21,22].
In the present work, three sets of mixed oxide catalysts, two binary oxides (Ce–Mn and Ce–Cu) and one ternary oxide (Ce–Mn–Cu), with various foreign metal loadings were synthesized via solution combustion synthesis (SCS). The catalysts were characterized by the N2 physisorption at − 196 °C, X-ray diffraction analysis, X-ray photoelectron spectroscopy, H2 temperature-programmed reduction, O2 temperature-programmed desorption and field emission scanning electronic microscopy. Their catalytic activity was tested towards VOC oxidation reactions, using ethylene and propylene as probe molecules. Indeed, the two hydrocarbons are representative of a wide group of VOCs and therefore possible precursors of carbonaceous matter.
The most active powder catalysts were then deposited on a silicon carbide (SiC)-based monolith via a novel in-situ SCS. The catalyzed monoliths were finally tested for the VOC oxidation in a bench-scale reactor.
2 Experimental
2.1 Catalysts Preparation
Pure cerium, manganese and copper oxides, as well as binary (Ce–Mn, Ce–Cu) and ternary oxide catalysts (Ce–Mn–Cu) were prepared through the solution combustion synthesis (SCS) [13, 19, 23,24,25]. The mixed oxide catalysts were prepared with different foreign metal contents: 5, 15 and 45 at.%. The notation is: Ce100−xMx, where M represents either Mn or Cu for the binary oxides, and Ce100−xMnx/2Cux/2 for the ternary oxides. Therefore, the list of powder catalysts prepared in the current work is: CeO2, Ce95Mn5, Ce85Mn15, Ce55Mn45, Mn2O3, Ce95Cu5, Ce85Cu15, Ce55Cu45, CuO, Ce95Mn2.5Cu2.5, Ce85Mn7.5Cu7.5, Ce55Mn22.5Cu22.5. During a typical synthesis, proper amounts of metal precursors, namely Ce(NO3)3·6H2O (Alfa Aesar); Mn(NO3)2·4H2O; Cu(NO3)2·3H2O (Sigma-Aldrich), were dissolved in 100 ml of deionized water along with a “fuel” (urea), whose amount was made in excess (200% of the stoichiometric amount). The amounts of precursors were defined to have nominal metal-to-cerium ratios.
The mixture was stirred vigorously at r.t. for 5 min. Then, the homogeneous aqueous solution was heated in oven at 650 °C for 30 min.
The most catalytically active powder samples (namely, Ce55Mn22.5Cu22.5 and Ce55Mn45) were washcoated in silicon carbide (SiC) monoliths [Céramiques Techniques et Industrielles s.a.s., 290 cpsi (cells per square inch), 3 cm in length and 2.54 cm in diameter], through an in-situ SCS with the same precursor mixtures used for the preparation of powder catalysts. The deposition was carried out by carefully injecting the precursor solution to the inner channels of the SiC monolith. Then, the monolith was placed in a pre-heated oven at a calcination temperature of 650 °C for 5 min. After a fast synthesis/calcination, the monolith was cooled down till r.t. and subsequently blown with compressed air to remove the undeposited powder from the channels. This procedure was repeated several times till the catalyst loading was equivalent to 15 wt%. The two monoliths are denoted as Ce55Mn45–SiC and Ce55Mn22.5Cu22.5–SiC.
2.2 Catalyst Characterization
The X-ray diffraction (XRD) patterns were measured on a X’Pert Philips PW3040 diffractometer using a Cu Kα radiation (2θ range 20° to 80°, step = 0.05° 2θ, time per step = 0.2 s). Such patterns were analyzed using the Powder Data File Database (PDF 2000, International Centre of Diffraction Data, Pennsylvania). The average size of the crystallites was determined through the use of the Scherrer formula, D = 0.9λ/(b cos θ), where λ is the wavelength of the Cu Kα radiation, b is the full width at half maximum (in radians), 0.9 is the shape factor considered for spherical particles and θ is the angle of the diffraction peaks.
The specific surface area (SBET), total pore volume (VP) and the average pore diameter (DP) were determined through the N2 physisorption analysis at − 196 °C (Micromeritics Tristar II 3020) on powders previously outgassed at 200 °C for 2 h to remove moisture/adsorbed water and atmospheric pollutants. The SBET was determined according to the Brunauer, Emmett and Teller (BET) method.
The catalyst morphology was studied through a field emission scanning electronic microscope (FESEM) Zeiss Merlin, Gemini-II column, using an extra high tension (EHT) of 3 kV, a working distance (WD) of 2.8 mm and a probe intensity of 120 pA. Additionally, the chemical composition of the catalysts was determined through EDS analysis (Oxford X-ACT) on two different areas/zones on the sample with a size/diameter of 5–25 µm. To analyze the distribution of different elements on the surface of the samples, EDS color coded mapping was also performed for the most active catalysts.
Temperature-programmed reduction/desorption analysis was realized in a ThermoQuest TPD/R/O 1100 analyzer, equipped with a thermal conductivity detector (TCD). The reducibility of the powder catalysts was studied by H2-TPR, in which a pretreatment was carried out before the analysis by treating 50 mg of the sample with helium (40 ml min−1) at 550 °C for 1 h. In the H2-TPR analysis, the catalyst was placed in a quartz tubular reactor and exposed to a reducing gas flow of 5 vol.% H2 in Ar (20 ml min−1). The analysis was conducted under a programmed heating from 50 to 800 °C with a ramp of 5 °C min−1 and the final temperature hold for 10 min. In the O2-TPD analysis, 50 mg of sample was initially pretreated under a pure O2 flow (40 ml min−1) at 550 °C for 30 min and then cooled down to 50 °C still under the O2 flow. Then, the TPD measurement was performed by heating the sample until 950 °C under a He flow (20 ml min−1) at a constant heating rate (5 °C min−1). For comparison purposes, three commercial MnOx samples (MnO2, Mn2O3 and MnO, from Sigma-Aldrich) were investigated under the same operating conditions.
XPS (X-ray photoelectron spectroscopy) measurements were performed on an XPS PHI 5000 Versa probe apparatus using the following conditions: band-pass energy of 187.85 eV, a 45° take off angle and a 100.0 μm diameter X-ray spot size. The curve-fitting was performed by means of Multipack 9.0 software.
2.3 Catalytic Activity Tests
Catalytic activity tests of powder samples were performed in a classical temperature-programmed oxidation setup, comprising a fixed-bed quartz U-tube reactor (ID = 4 mm) to host the catalyst powder. The reactor was heated by a PID-controlled furnace and the reactor bed temperature was measured by a K-type thermocouple.
Before the test, 0.1 g of powder catalyst was pretreated to remove the adsorbed species. During the pretreatment, He was fed (flow rate = 15 ml min−1) for 1 h, maintaining a constant temperature of 150 °C. The catalytic test started with feeding to the reactor a gaseous reactive mixture containing 500 ppm of VOC (either ethylene or propylene) and 10 vol.% of O2 (with N2 to balance).
During the test, a gas hourly space velocity (GHSV) of 20,000 h−1 and a catalyst weight-to-volumetric flow rate ratio (W/F) of 0.065 g h l−1 was maintained. The temperature was raised in the range of 50–350 °C, and an isothermal step was performed every 50 °C. The outlet gas composition was measured by using non-dispersive infrared (NDIR) analyzers (ABB Uras 14). To confirm the reproducibility of the catalytic tests, three consecutive catalytic tests were performed, keeping the same operative conditions. In order to perform the catalytic tests under wet conditions (RH 100%), the reactant gas flow was saturated by bubbling it inside a vessel containing water at 20 °C before flowing it to the U-tube reactor.
To test the activity of the catalytic monoliths (Ce55Mn45–SiC and Ce55Mn22.5Cu22.5–SiC), a stainless-steel reactor and a horizontal furnace (60 cm-long) were used. To optimize the gas flow distribution in the monolith, the prepared monolith was fixed in a hollowed cordierite monolith (3.5 cm in diameter). The temperatures (inlet and outlet) of the catalyzed monolith were measured by two K-type thermocouples. Before the tests, the monolith was outgassed with He (flowrate = 300 ml min−1) at 150 °C for 1 h. Then, a gas mixture containing 500 ppm of VOC (either ethylene or propylene) and 10 vol.% of O2 (with N2 to balance), was fed to the reactor by mass-flow controllers. GHSV = 9000 h−1 and W/F = 0.031 g h l−1 were maintained during the tests; the temperature was raised from 100 to 620 °C at a heating rate of 5 °C min−1. The outlet gas composition was then measured by the NDIR. Each test was repeated twice to evaluate the reproducibility. Additionally, time on stream (TOS) tests were performed to study the stability of the catalyzed monolith.
3 Results and Discussion
3.1 Material Textural Properties
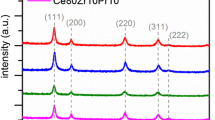
In Fig. 1, the XRD patterns of the synthesized catalysts are shown. For pure ceria, the diffraction peaks correspond to the fluorite structure of cerium (IV) oxide (reference code 01-081-0792), with a face-centered-cubic-structure containing octahedral and hexahedral holes filled with oxygen ions [17]. It can be seen that for the binary (Ce–Mn; Ce–Cu) and ternary (Ce–Mn–Cu) oxide catalysts, the most intense peaks corresponding to ceria fluorite structure are still observed for the samples even with a high content of manganese and/or copper. However, in the diffractograms of the binary and ternary oxides with 45% metal content were observed the peaks corresponding to pure oxides of copper or manganese (CuO ref. code 00-045-0937; Mn2O3 ref. code 01-078-0390) and mixed copper-manganese oxide (Cu1.5Mn1.5O4 ref. code 01-070-0260), thus suggesting the presence of extra-framework species (metal oxides) from the lattice. It can also be seen that the XRD peak broadens as the metal content increases. This behavior seems related to the fact that the content of small and defect-rich crystallites in the samples is increased [26]. The latter condition, indeed, could favor the formation of smaller crystallites, with an average size of 12 nm (estimated via the Scherrer’s formula). This means that elevated percentages of foreign metals in the solids lead to smaller crystallites, as shown the Fig. S1. On the other hand, strain effects along with structural defects could occur when foreign metals (= different radii) are introduced into the ceria framework.
Table 1 summarizes the various textural properties of the samples as derived from both XRD and N2 physisorption analysis. As a whole, The BET surface areas were observed to have values in the range of 8–48 m2 g−1. Specifically, at lower foreign metal contents, the specific surface areas for mixed oxide samples increase with the Mn/Cu-loadings. On the other hand, at higher foreign metal contents worse textural properties can be observed. An exception to this trend occurs for the Ce95Mn2.5Cu2.5 sample, exhibiting a smaller BET surface area (8 m2 g−1), probably due to the formation of big crystallites (Table 1; Fig. S1). Conversely, comparable pore volumes (VP = 0.02–0.12 cm3 g−1) and pore diameters (DP = 11–24 nm) can be observed for the prepared samples.
Figure 2 shows the morphological properties of the samples. Most of them exhibit a globular/sponge-like morphology, which is the feature of the parent oxide (CeO2). In the case of Ce–Mn oxides, the size of such agglomerates seems to increase until the Mn content reaches 15 at.%. At higher Mn contents, the agglomerate size decreases. The previous behavior of the superficial features seems to follow the trend of the specific surface area reported in Table 1. The diameter of the globular agglomerates that constitute the Ce–Cu samples (Ce95Cu5 and Ce85Cu15) seems comparable, while Ce55Cu45 exhibits smaller spherical structures. Both the Ce95Mn2.5Cu2.5 and Ce85Mn7.5Cu7.5 samples show small agglomerates and compact structures with visible grain boundaries. Their predominantly non-porous structures confirm the low specific surface areas observed for the ternary oxide samples. The pure copper oxide sample (CuO) exhibits a compact structure with low porosity, while the pure manganese oxide (Mn2O3) demonstrates small pores distributed internally and externally on the surface. The EDS element maps of the catalysts containing 45% foreign metals are shown in Fig. S3. The analysis performed on some planar zones on the samples showed a uniform distribution of the elements on the catalytic surface. The atomic percentages obtained by the EDS analysis are shown in Table 2. The results show that the measured atomic percentages of the elements present in the sample are in fair agreement with the nominal percentages.
3.2 H2-TPR and O2-TPD Analysis
The H2-TPR profiles of the synthesized catalysts are shown in Fig. 3. The reduction of CeO2 started below 100 °C and was characterized by two main peaks: the one with a lower intensity at 194 °C and the one with a more remarkable intensity at 517 °C. According to the literature, the peak centered at higher temperature can be ascribed to the reduction of Ce species, while the peak appeared at lower temperatures corresponds to the reduction of surface oxygen species [19, 27]. Additionally, the signal occurring at higher temperature (above 600 °C) can be attributed to the reduction of cerium species in the bulk phase.
The reduction of copper oxide particles was characterized by one sharp peak centered at 266 °C, consistent with the literature [21, 28]. The reduction of Mn2O3 was characterized by two overlapping reduction peaks. The first one appeared at 325 °C and was attributed to the reduction step of Mn2O3 → Mn3O4, and the second one was centered at 444 °C. The deconvolution of these two peaks revealed a total H2 uptake of about 9.22 mmol g−1, which is higher than the theoretical H2 uptake of pure Mn2O3 (6.33 mmol g−1). Such value is lower than the corresponding theoretical uptake when all the manganese is present in the Mn4+ form (11.50 mmol g−1). This means that the aforementioned peaks correspond to the reduction of both Mn4+ and Mn3+ species, as evidenced by the XPS analysis. Therefore, the reduction pathway for the Mn2O3 sample, as reported in the literature, is MnO2 → Mn2O3 → Mn3O4 → MnO [13, 29].
The H2-TPR profiles of the binary Ce–Mn samples, as seen in Fig. 3a, show a multitude of overlapping reduction peaks at lower temperatures. In general, the H2 uptake and catalyst reducibility increases with Mn-contents. The reduction profiles of the Ce95Mn5 and Ce85Mn15 catalysts show that the insertion of manganese into ceria lattice enhances the reducibility at lower temperatures, thus shifting the main reduction peak from 517 to 185 °C. In the case of Ce55Mn45, the reduction profile and the H2 uptake change markedly and this can be due to the presence of manganese oxide species interacting with ceria.
The reduction profiles of the Ce–Cu group of samples are shown in Fig. 3b. It can be noticed that the presence of Cu in the samples promotes the reducibility at lower temperatures. The Ce95Cu5 and Ce85Cu15 samples are characterized by two peaks, one appeared at 150 °C and another one at 169 °C (182 °C for the samples with higher copper contents). The peak at the lower temperature was attributed to Cu species in the lattice interacting with cerium (Ce–O–Cu), while the peak at the higher temperature can be assigned to highly dispersed CuOx clusters interacting with ceria [19, 27, 28, 30]. For the Ce55Cu45 sample an additional peak appears at higher temperature, in which segregation of copper oxides and therefore further formation of crystalline CuO had presumably occurred during the synthesis (as demonstrated by the XRD analysis). Therefore, the peak of Ce55Cu45 occurring at 209 °C is due to the reduction of CuO clusters dispersed on ceria [19, 27, 28, 30]. Finally, the surface reducibility of the Ce–Cu systems increases as a function of the Cu content, as follows: Ce95Cu5 < Ce85Cu15 < Ce55Cu45.
As seen in Fig. 3c, the reduction behavior of the Ce–Mn–Cu samples is similar to that of binary Ce–Cu samples, which generally exhibit two reduction peaks at about 150 °C and 180 °C. This means that the copper species gives the major contribution to the overall catalyst reducibility. However, the presence of manganese species in the ternary oxide samples seems to induce a slight shift of the reduction peaks attributed to the copper species [31, 32]. The two reduction peaks of Ce95Mn2.5Cu2.5 and Ce85Mn7.5Cu7.5 were attributed to the reduction of isolated copper species interacting with cerium and manganese in the lattice (149 and 154 °C), and to the reduction of finely dispersed CuOx clusters interacting with the mixed oxide (177 and 182 °C). The H2-TPR profile of the Ce55Mn22.5Cu22.5 catalyst consists of several overlapping peaks, which in turn render more difficult the analysis of the reduction behavior. The peak at ca. 157 °C was attributed to the reduction of isolated copper species, which may be enhanced by the presence of manganese species. On the other hand, the signal at about 207 °C can be assigned to the combined reduction of the Cu1.5Mn1.5O4 spinel and the CuOx clusters, both interacting with ceria. Finally, the peak centered at 232 °C was ascribed to the reduction of CuO species.
The trends of the low-temperature reduction peaks (Fig. 3d) show a possible role of the foreign metals in the ceria-based samples. It is evident that the presence of Mn/Cu species into the ceria shifts the reduction peaks at lower temperatures compared to pure ceria (= easier surface reducibility). Interestingly, for both the 5 and 15 at.% doped samples, the reduction peaks take place at the lowest temperature (about 150 °C). This finding can be observed also in terms of H2-uptake and may be related to the insertion of the metals in ceria’s lattice with the formation of solid solutions. On the other hand, at higher metal contents the surface reduction occurs at higher temperatures, due to the presence of segregated Mn and Cu oxides as evidenced by the XRD data.
Finally, the deconvolution of the H2-TPR profiles revealed the involvement of ceria during the reduction. Table 3 reports the total H2 uptake of all the prepared catalysts and the percentage of Ce4+ species reduced during the analysis, on the assumption that the Mn and Cu species were initially present in the sample in the + 3 and + 2 oxidation states, respectively, and that they were reduced to Mn2+ and Cu0. It can be seen that the overall H2 uptake of the catalysts is generally higher than the stoichiometric uptake by the reduction of the manganese and/or copper species in the catalysts. In addition, the reduction of the Ce4+ species, which represents the participation of ceria in the reduction, seems to increase with foreign metal loading. This indicates a positive impact of both Mn and Cu as the foreign metals on improving the reducibility of ceria, possibly through the interactions among the oxides in the catalyst.
Figure 4 summarizes the results obtained from the O2-TPD analysis. The O2-TPD profiles of the binary Ce–Mn oxide samples are shown in Fig. 4a. The TPD profile of Mn2O3 shows one intense peak appeared at 826 °C. The estimated quantity of oxygen desorbed during the analysis, which corresponds to the peak integral area (1.09 mmol g−1), matches the theoretical quantity of oxygen released in the reduction step Mn2O3 → Mn3O4 (1.06 mmol g−1). Similar value has also been reported elsewhere [13, 15]. The pure ceria sample (CeO2) did not show any relevant oxygen desorption under the same analysis condition. To better analyze the oxygen desorption characteristics for the pure ceria sample, an additional analysis was performed under the conditions reported in the literature [33] and results are shown in Fig. S4. The peak appeared at approximately 850 °C, which corresponds to the desorption of superficial oxygen species. The presence of manganese in the Ce–Mn samples generally endorses an improvement in the catalyst oxygen storage capacity. According to the literature, the presence of Ce species in the solid promotes the higher oxidation state of Mn species at moderate temperatures [34]. The TPD profiles for the Ce–Mn–Ox samples show complex patterns (Fig. 4a). As a whole, the oxygen desorption at low temperatures (< 400 °C) can be attributed to the release of chemisorbed (Oα-type) species (magnification in Fig. S5), whereas the peaks at higher temperatures can be ascribed to lattice oxygen (Oβ-type species) [35]. Specifically, the Ce55Mn45 sample demonstrates an intense peak centred at 638 °C, readily assigned to the reduction of Mn4+ species. On the other hand, different profiles can be observed for both Ce85Mn15 and Ce95Mn5 samples. For the latter samples, indeed, additional signals appear above 800 °C, thus suggesting the presence of reduced Mn3+/Mn2+ species, along with Mn4+ cations. These results are in fair agreement with the XPS data (vide infra).
At lower temperature, the presence of multiple peaks suggests the presence of an abundant population of chemisorbed oxygen. These TPD results are in fair agreement with those derived from a set of commercial manganese oxides (MnO2, Mn2O3 and MnO) included in Fig. S6: For the MnO2 sample the reduction of Mn4+ to Mn3+ occurs at about 580 °C with an oxygen release of 2.63 mmol g−1 (step MnO2 → Mn2O3). This value is in fair agreement with the theoretical (calculated) oxygen release (2.87 mmol g−1); the signal at higher temperature (ca. 811 °C) corresponds to the reduction of Mn3+ species to Mn2+. The integral area of the latter peaks evidenced an oxygen release of 1.13 mmol g−1 (step Mn2O3 → Mn3O4). The theoretical oxygen release for this step is about 1.06 mmol g−1. Similar results occur with the reduction of Mn2O3 (peak at ca. 806 °C, oxygen release = 1.13 mmol g−1) thus confirming that Mn3+ species can be reduced at about 800 °C. On the other hand, for the MnO sample there are not peaks in the TPD pattern, suggesting that Mn2+ species do not change their oxidation state during the analysis. Figure 4b also shows the O2 desorption profiles for the CuO sample and the Ce–Cu catalysts. The reduction profile of CuO shows a single peak at 927 °C. The estimated quantity of oxygen desorbed during the analysis (3.13 mmol g−1) is in accordance with the theoretical quantity of oxygen released in the reduction of Cu2+ to Cu+ (3.14 mmol g−1).
The TPD profiles for the Ce–Cu systems showed an enhancement of the Oα species when the percentage of copper was increased. Thus, the presence of Cu species improves the abundancy of chemisorbed oxygen species on CeO2-based samples. Similarly, the Oβ desorption rate seems to increase with the Cu content. The desorption peak of Ce55Cu45 is similar to that of pure CuO, although it is lower in intensity and centered at a lower temperature (ca. 887 °C). Such a similarity has led us to suggest that the desorption is probably related to the release of lattice oxygen of the CuOx phases (i.e. Cu2+ → Cu+). Meanwhile, in the case of Ce85Cu15 and Ce95Cu5, the main desorption peaks are centered at slightly lower temperatures and are lower in intensity, most likely due to the decreasing amount of Cu2+ species. In addition, the wide peaks occurring between 400 and 700 °C were assigned to Oβ released from the mixed Ce–O–Cu lattice.
Figure 4c shows that the co-presence of manganese and copper oxides in the ternary oxide samples enhances significantly the release of Oα-type species. The peaks centered at around 750 °C and 823 °C were ascribed to the release of Oβ species. However, such ascription was not limited to weaker metal–oxygen bonds, since according to the O2-TPD analysis of CeO2 and Mn2O3, the peak ascribed to the weakening of Ce–O and Mn–O bonds was centered at a similar position. Moreover, as evidenced by the TPD profiles of the Ce–Cu samples, the desorption of Ce–O–Cu lattice oxygen may take place at a similar temperature.
3.3 X-ray Photoelectron Spectroscopy
Figure 5a shows the deconvoluted XPS spectra of the prepared samples in the O 1s core level. Three peaks that refer to various oxygen species were identified for most samples. The peak appeared in the binding energy (BE) range of 529.0–529.5 eV was attributed to the lattice-like oxygen species (Oβ-species), which are bonded to Ce [36], while that appeared at 529.8 eV was attributed to the lattice-like oxygen species bonded to manganese or to copper [37, 38]. The peak between 531.1 eV and 531.5 eV was attributed to surface oxygen species (Oα-species) like O−, or O2− and OH groups [39, 40]. The low-intensity peak appeared at higher BE range (between 533.2 and 534.3 eV) and it was observed in the spectra of the mixed oxide samples. This peak was attributed to the presence of more electrophilic Oα species like weakly adsorbed oxygen species [15, 41, 42]. As seen in Table 4, the Oα/Oβ ratio for the Ce–Mn catalysts increases with Mn content, while that for copper-containing samples (i.e. Ce–Cu and Ce–Mn–Cu) seems to reach a minimum at the foreign metal content of 15 at%. For the mixed oxide samples, the abundance of Oα species is higher than that of CeO2 but lower than those of Mn2O3 and CuO. The trend of the relative abundances of Oα among the mixed oxide catalysts observed via XPS analysis corroborates that of the oxygen release observed through the O2-TPD analysis, especially at low temperatures (< 400 °C), as shown in Fig. S5.
Figure 5b shows the Ce 3d XP spectra of all the ceria mixed oxides. Generally, the spectra are composed of a series of spin–orbit doublets “V” and “u”, related to/located in the 3d5/2 and 3d3/2 regions, respectively. The common BE positions of such peaks, according to the current analysis, are as follows: V0 at 882.0 eV; V1 at 884.6 eV; V2 at 888.4 eV; V3 at 898.0 eV; and u0 at 900.6 eV; u1 at 902.0 eV; u2 at 907.1 eV and u3 at 916.3 eV. These peaks are related to cerium species with various oxidation states and were used to calculate the abundances of Ce4+ and Ce3+ on the surface. The doublets (V0, u0), (V2, u2) and (V3, u3) were assigned to Ce4+, while the doublet (V1, u1) was assigned to Ce3+ [39, 43]. The relative abundances of these two species in the samples are summarized in Table 5. It can be seen that the atomic percentage of Ce3+ observed for the mixed oxide samples is higher than that of CeO2. This indicates that the insertion of Mn and/or Cu in the ceria can produce a distortion in the fluorite framework. This leads to an increase in the abundancy of defects and therefore to the formation of new Ce3+ sites [39, 44, 45].
Figure 5c shows the Mn 2p XP spectra of the Mn-containing catalysts. The spin orbit peaks appeared in the range of 647.5–657.5 eV correspond to the 2p1/2 level, while those appeared between 638.5 and 647.5 eV correspond to the 2p3/2 level. The deconvolution of the 2p3/2 spectra has been used in many investigations to estimate the relative abundances of the Mn4+, Mn3+ and Mn2+ species in the sample [13, 37]. The presence of these Mn species was observed for the Mn2O3 sample (Mn3+ are the most abundant ones). The relative amounts of Mn ions derived from the Mn 2p spectra deconvolution are summarized in Table S1. As a whole, it can be seen that the abundances of Mn species depend on the Mn content. In fact, the Ce55Mn45 sample has the highest amount of Mn4+ species among the samples, whereas for both the binary (Ce95Mn5 and Ce85Mn15) and ternary oxides (Ce95Mn2.5Cu2.5 and Ce85Mn7.5Cu7.5), the abundancy of Mn2+ species is significantly higher.
Figure 5d finally shows the Cu 2p XP spectra of the copper-containing samples. The spin orbit peaks appeared in the range of 930.0–940.0 eV belong to the 2p3/2 level, whereas those in the range of 950.0–960.0 eV belong to the 2p1/2 level. The deconvolution of the latter is normally used to identify the copper species in the sample as well as the shake-ups related to those species [38, 46, 47]. In the case of the CuO, Cu2+ strong satellites at 942.5 and 962.5 eV were identified. Therefore, the major peak of 2p1/2 region was unarguably ascribed to Cu2+ and can be deconvoluted to two peak components: one centered at 933.6 eV and attributed to Cu species bonded to lattice oxygen (Cu–O), and another one centered at 935.2 eV and attributed to the copper hydroxide and/or copper carbonate species [38, 46]. The results for the deconvolution of the Cu 2p spectra are shown in Table S2. It can be observed that, in the case of Ce55Cu45 and Ce55Mn22.5Cu22.5, the predominant oxidation state of Cu was + 2, most likely in the form of copper species bonded to lattice oxygen as well as to hydroxides/carbonates. The rest of the copper containing catalysts demonstrates a shift of the major 2p1/2 peak to a lower binding energy (932.5 eV), thus indicating the presence of Cu+ species. Such a reduced species most likely originates from a reduction induced by an interaction among the metal cations [19, 38].
Finally, Table 6 shows the atomic percentages of the elements in the prepared samples. As a whole, it appears that XPS results are in fair agreement with EDS data (Table 2), thus suggesting a good dispersion of the foreign metals for both the surface and bulk phases. As shown in the new Table S3 (Supporting Information), the differences (in terms of ∆Cu and ∆Mn) between the two techniques are low and so it is not possible to argue any whole enrichment or diminution of the foreign metals on the sample surfaces with respect to the bulk compositions.
3.4 Catalytic Activity of Powder Catalysts
3.4.1 Propylene Oxidation
As a whole, Fig. 6 shows the conversion of propylene (sections a, c and e) and ethylene (sections b, d and f) as a function of temperature for the prepared samples. The light-off temperature for the non-catalytic oxidation of propylene was observed at ca. 300 °C and the reaction reached a conversion of approximately 4% at 350 °C. In the presence of catalyst, the propylene oxidation reaction occurred at much lower temperatures and reached a complete conversion over several catalysts below 350 °C.
Table 7 summarizes the catalytic performances in terms of temperatures at which 10%, 50% and 90% propylene conversion to CO2 are obtained (T10%, T50% and T90%, respectively) as well as the reaction rates. Observing the T10%, T50% and T90% values, the performances for pure oxides follow this trend: CeO2 (worst activity) < CuO < Mn2O3. The presence of foreign metals in the mixed oxides improves the performances in propylene oxidation, thus indicating possible interactions between Ce, Mn and/or Cu species or among different oxide phases on the surfaces (as revealed in the XRD analysis).
The substitution of Ce species with Mn/Cu atoms may enhance the oxygen defects in the samples, thus improving the mobility of lattice oxygen and consequently improving the reducibility of the samples, as evidenced by the TPR analysis. For each catalyst group, it was observed that both catalytic performance and catalyst reducibility increased with metal loading, thus confirming the beneficial effect of metal cation substitution. On the other hand, the crystallite size, as evidenced by XRD analysis, decreased with metal loading. Interestingly, there is a good correlation between the performances of the mixed oxide catalysts and their crystal size: the lower the crystal sizes the better the performances (Fig. S7).
In terms of reaction rates (measured at 150 °C), mixed oxide catalysts generally exhibited better activities than CeO2 and, in some cases, than Mn2O3 or CuO. The Ce55Mn45 sample, is the most intrinsically active catalysts and a complete propylene conversion over this catalyst can be achieved at 250 °C. Such a high performance is most likely derived from an interaction between MnOx and CeOx phases that further contributes to the improvement of redox properties of the catalyst. Then, a performance trend for the prepared systems can be drawn: Ce–Cu < Ce–Mn–Cu < Ce–Mn.
For the first time to our knowledge, these results confirm that the presence of Mn species into ceria-based catalysts prepared by SCS may promote the total oxidation of propylene.
Concerning the oxidation mechanism of propylene over metal oxide catalysts, many researchers argued that VOC molecules can be first adsorbed on the catalytic surface and then a symmetric allylic intermediate is formed as a consequence of the H-abstraction. Subsequently, the oxidation occurs on the catalyst surface by the release of lattice oxide (O2−) and its consequent insertion in the chemical structure of the intermediate, thus forming an allyl alkoxide. Further H-abstraction takes place and the carbonyl bond leads to the formation of an aldehyde, which is then totally oxidized. Thus, the VOC oxidation over ceria-based catalysts seems to occur via a Mars–van Krevelen-type mechanism (Scheme 1) [19]. However, the presence of catalytic surfaces with abundant weakly-bonded oxygen species is beneficial for the oxidation process since organic molecules can be adsorbed and the reaction take place. Similarly, spillover phenomena may occur on the catalytic surface thus enhancing the whole reactivity [48,49,50].
Redox cycle of ceria-based systems (M″ and open square box represent the foreign metal, and the oxygen vacancy, respectively). Adapted from [19]
Figure 7 (sections a, c and e) shows the results derived from the stability tests of the most active catalyst for each set (i.e. Ce55Cu45, Ce55Mn45 and Ce55Mn22.5Cu22.5) in propylene oxidation. In all cases, reproducible results were achieved after three consecutive runs. In the case of Ce55Cu45, an early increase of COx at 150 °C can be related to a fast release of CO2 at low temperature conditions. Moreover, the best catalyst (Ce55Mn45) was tested under the presence of humidity (RH 100%). Although worse catalytic performances were observed in the present case (Fig. S8), it appears that stable catalytic results can be observed for consecutive catalytic runs. This suggests that ceria-based catalysts acting in wet-conditions are less performing. This finding seems due to different causes, e.g. thermal phenomena localized on the catalytic particles, lower accessibility of active sites, etc.
Further kinetic experiments for the propylene oxidation over the Ce55Mn45 catalyst were performed varying the inlet VOC concentration (200, 400, 600 and 900 ppm) at 150 °C. As a whole, it was observed a positive (linear) dependence of the oxidation reaction rate (μmolVOC h−1) on the VOC concentration (Fig. S9.A).
3.4.2 Ethylene Oxidation
Figure 6 (sections b, d and f) shows the catalytic tests with ethylene oxidation. At 350 °C, the non-catalytic reaction reached < 1% ethylene conversion. In Table 7, it can be seen that the catalyzed ethylene oxidation also resulted in higher reaction temperatures than the catalyzed propylene oxidation and this demonstrates the lower ability of ethylene to be oxidized due to its higher stability. The catalytic performances for pure oxide samples (CeO2, Mn2O3 and CuO) follow the same trend of the performances achieved in propylene oxidation: CeO2 < CuO < M2O3. The mixed oxide samples provided better performances than ceria and their catalytic activities increased with the foreign metal loadings, as well as for the propylene oxidation. Possible interactions between Ce, Mn and/or Cu species in the samples or among different phases are equally beneficial for ethylene oxidation.
As shown in Table 7, the Ce55Mn22.5Cu22.5 sample is the most active catalyst for the ethylene oxidation at 160 °C followed by the Ce55Mn45 (2.75 and 1.21 μmol h−1 m−2, respectively). Interestingly, the total conversion of ethylene over the Ce55Mn22.5Cu22.5 sample was achieved at about 250 °C. These findings are possibly due to the interaction between the different active phases, as revealed on the Ce55Mn22.5Cu22.5 sample (vide supra). For the first time to our knowledge, these results confirm that the presence of both Mn and Cu species into ceria-based catalysts prepared by SCS may promote the total oxidation of ethylene. Like in propylene oxidation and in many other VOC oxidations, ethylene oxidation may also be kinetically modelled via a redox-type cycle (i.e. Mars–van Krevelen reaction mechanism), which involves the participation of surface oxygen species. Particularly in the case of ethylene, previous studies have shown that the oxidation mechanism involves a mild oxidation of the molecule on the surface of ceria and the formation of intermediate formate species, which are consequently oxidized to CO and CO2 [51, 52].
A series of stability tests were equally performed with ethylene oxidation over the most active catalyst in each group (i.e. Ce55Cu45, Ce55Mn45 and Ce55Mn22.5Cu22.5) and the results are shown in Fig. 7 (section b, d and f). After three consecutive runs, the complete ethylene conversion was achieved over powder catalysts at 350 °C.
As already mentioned for the propylene oxidation, the best catalyst (Ce55Mn22.5Cu22.5) was tested under wet-conditions (Fig. S8). Although worse catalytic performances were observed at RH 100% rather than in dry conditions, comparable results were achieved for two consecutive catalytic runs in the presence of humidity. This further confirm the findings reported for the oxidation of propylene (vide supra).
Additionally, kinetic studies carried out over the Ce55Mn22.5Cu22.5 sample with different inlet ethylene concentrations (200, 400, 600 and 900 ppm) at 160 °C confirm that the oxidation rate does not depend on VOC inlet concentration (Fig. S9.B), thus suggesting a zero order-type mechanism respect to the ethylene.
3.5 Catalytic Activity of the Catalyzed Monoliths
With the aim of evaluating the applicability of the most active catalysts studied in this work for industrial purposes, both the Ce55Mn45 and Ce55Mn22.5Cu22.5 catalysts were deposited on SiC monoliths via in-situ synthesis. Figure 8 shows various FESEM images of a cross-sectional cut of an inner Ce55Mn22.5Cu22.5–SiC channel taken at various magnifications. The images on the right were taken in backscattered electron mode to better discriminate the catalytic washcoat (lighter color) from the SiC grains (darker color). The micrograph on the center shows the presence of catalytic washcoat on the inner wall of the monolith channel and confirms the effectiveness of the in situ synthesis in forming a thin catalytic layer (thickness of 326 nm in average) in the channel. Figure S3 (Section D) shows an EDS map that was performed on a representative zone of the sample. The map shows an even distribution of the elements over the surface.
Figure 9 (sections a and b) shows the stability tests with either propylene or ethylene oxidation over the Ce55Mn45–SiC monolith. In both reactions, the catalytic monolith is effective for the VOC oxidation. The catalytic reaction reached a complete propylene and ethylene conversion at around 500 °C and 600 °C, respectively, while the non-catalytic (thermal) oxidation is achieved at higher temperatures (ca. 670 °C). The catalyzed monolith also demonstrated comparable catalytic performances for three catalytic runs, thus confirming a good catalyst stability for both the oxidation reactions. To further verify the stability of Ce55Mn45–SiC, several time-on-stream (TOS) measurements were performed under two different isothermal conditions: (1) at higher temperature (470 and 590 °C for propylene and ethylene oxidation, respectively), at which a nearly complete VOC conversion was achieved and (2) at lower temperature (372 and 471 °C for propylene and ethylene oxidation, respectively), at which ca. 50% conversion can be achieved.
Similarly, Fig. 9 (sections c and d) shows the stability tests with either propylene or ethylene oxidation over the Ce55Mn22.5Cu22.5–SiC monolith. As a whole, it was observed that the reaction mediated by Ce55Mn22.5Cu22.5–SiC mostly occurred at lower temperatures than that mediated by Ce55Mn45–SiC. The catalyst stability was also appreciable after three consecutive runs.
A study of Ce55Mn45–SiC and Ce55Mn22.5Cu22.5–SiC was carried out under two different isothermal conditions to investigate the stability at a nearly complete conversion and at 50% conversion. As shown in Fig. 10, fairly stable propylene/ethylene conversions can be observed over a TOS of 4 h, thus suggesting promising structural and catalytic stability of the monoliths. A long term-stability study under isothermal conditions with the Ce55Mn22.5Cu22.5–SiC has confirmed a stable catalytic behaviour for two catalytic runs over a time-on-stream of 12 h (Fig. S10).
In order to investigate the catalytic behavior of the monoliths under different operating conditions, additional experiments were done at various GHSV (namely, 1000, 4335, 9000 and 19,000 h−1) as reported in Fig. 11. As a whole, a general trend occurs between the GHSV values and the catalytic performances for both the propylene and ethylene oxidation: the higher the GHSV the lower the VOC conversion. Specifically, the performances of the monolith-supported catalysts are closer to those of powder samples at 1000 h−1, as a consequence of the higher residence time of the reactants into the channels. In fact, the worst results of catalyzed monoliths can be obtained at 19,000 h−1. Finally, in order to evaluate the catalytic activity of the monoliths at the same W/F of powder catalysts, further experiments were conducted at 4335 h−1. In the latter case, however, the performances of the monoliths are worse than those achieved for powder catalysts (see Fig. 6). This finding suggests that both the different catalytic set-up and fluid dynamics play a key role on the whole activity [53, 54]. These findings suggest that it is possible to develop scaled-up catalytic systems (wash-coated monoliths) via a novel in-situ SCS, taking in consideration the chemical composition of powder catalysts.
4 Conclusions
In this work, several mixed oxide catalysts were synthesized via the solution combustion synthesis (SCS): two sets of binary oxides (Ce–Mn and Ce–Cu) and one set of ternary oxides (Ce–Mn–Cu), with various metal contents (5, 15 and 45 at.%). The results confirmed that the Ce55Mn45 was the most performing catalyst for total oxidation of propylene, thanks to possible interactions between MnOx and CeOx species. For total oxidation of ethylene, the ternary oxide Ce55Mn22.5Cu22.5 was the most active catalyst, likely due to the interactions among CeOx, MnCuOx and CuOx species. In both cases, this catalytic improvement can be due to the enhanced reducibility of the catalysts.
Finally, two catalyzed monoliths (Ce55Mn45 and Ce55Mn22.5Cu22.5) were prepared through a novel in-situ SCS and their performances were tested in a bench-scale reactor. The catalytic results confirmed stable performances in terms of VOC oxidation of either propylene or ethylene for a time-on stream of 4 h.
Change history
16 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10562-021-03708-3
References
eCFR—Electronic Code of Federal Regulations Title 40—Chapter I—Subchapter C—Part 51—Subpart F. https://www.ecfr.gov/cgi-bin/text-idx?SID=9f2ffa73f1b399778c0c38af954be221&mc=true&node=sp40.2.51.f&rgn=div6. Accessed 3 May 2019
European Parliament and Council (2004) Directive 2004/42/CE on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain paints and varnishes and vehicle refinishing products and amending Directive 1999/13/EC. Off J Eur Union 10
Koppmann R (2007) Volatile organic compounds in the atmosphere. Blackwell Pub, Chicago
Bloemen HJT, Burn J (1993) Chemistry and analysis of volatile organic compounds in the environment. Springer, New York
Ramírez N, Cuadras A, Rovira E et al (2012) Chronic risk assessment of exposure to volatile organic compounds in the atmosphere near the largest Mediterranean industrial site. Environ Int 39:200–209. https://doi.org/10.1016/J.ENVINT.2011.11.002
Sarigiannis DA, Karakitsios SP, Gotti A et al (2011) Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ Int 37:743–765. https://doi.org/10.1016/J.ENVINT.2011.01.005
U.S. Environmental Protection Agency, Office of Air and Radiation (1992) Control techniques for volatile organic compound emissions from stationary sources
Moretti EC, American Institute of Chemical Engineers, Center for Waste Reduction Technologies. (2001) Practical solutions for reducing volatile organic compounds and hazardous air pollutants. Center for Waste Reduction Technologies, American Institute of Chemical Engineers
Kamal MS, Razzak SA, Hossain MM (2016) Catalytic oxidation of volatile organic compounds (VOCs)—a review. Atmos Environ 140:117–134. https://doi.org/10.1016/J.ATMOSENV.2016.05.031
Spivey JJ (1987) Complete catalytic oxidation of volatile organics. Ind Eng Chem Res 26:2165–2180. https://doi.org/10.1021/ie00071a001
Fino D, Bensaid S, Piumetti M, Russo N (2016) A review on the catalytic combustion of soot in Diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl Catal A Gen 509:75–96. https://doi.org/10.1016/J.APCATA.2015.10.016
Zhang Z, Jiang Z, Shangguan W (2016) Low-temperature catalysis for VOCs removal in technology and application: a state-of-the-art review. Catal Today 264:270–278. https://doi.org/10.1016/j.cattod.2015.10.040
Piumetti M, Fino D, Russo N (2015) Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl Catal B Environ 163:277–287. https://doi.org/10.1016/j.apcatb.2014.08.012
Kim SC, Shim WG (2010) Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl Catal B Environ 98:180–185. https://doi.org/10.1016/j.apcatb.2010.05.027
Santos VP, Pereira MFR, Órfão JJM, Figueiredo JL (2010) The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl Catal B Environ 99:353–363. https://doi.org/10.1016/j.apcatb.2010.07.007
Heynderickx PM, Thybaut JW, Poelman H et al (2010) The total oxidation of propane over supported Cu and Ce oxides: a comparison of single and binary metal oxides. J Catal 272:109–120. https://doi.org/10.1016/j.jcat.2010.03.006
Trovarelli A (2002) Catalysis by ceria and related materials. Imperial College Press, London
Ruiz Puigdollers A, Schlexer P, Tosoni S, Pacchioni G (2017) Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal 7:6493–6513. https://doi.org/10.1021/acscatal.7b01913
Piumetti M, Bensaid S, Andana T et al (2017) Cerium–copper oxides prepared by solution combustion synthesis for total oxidation reactions: From powder catalysts to structured reactors. Appl Catal B Environ 205:455–468. https://doi.org/10.1016/j.apcatb.2016.12.054
Delimaris D, Ioannides T (2008) VOC oxidation over MnOx–CeO2 catalysts prepared by a combustion method. Appl Catal B Environ 84:303–312. https://doi.org/10.1016/j.apcatb.2009.02.003
Delimaris D, Ioannides T (2009) VOC oxidation over CuO–CeO2 catalysts prepared by a combustion method. Appl Catal B Environ 89:295–302. https://doi.org/10.1016/j.apcatb.2009.02.003
Lu H, Kong X, Huang H et al (2015) Cu–Mn–Ce ternary mixed-oxide catalysts for catalytic combustion of toluene. J Environ Sci 32:102–107. https://doi.org/10.1016/J.JES.2014.11.015
Yu P, Zhang X, Chen Y, Ma Y (2010) Solution-combustion synthesis of ε-MnO2 for supercapacitors. Mater Lett 64:61–64. https://doi.org/10.1016/J.MATLET.2009.10.007
González-Cortés SL, Imbert FE (2013) Fundamentals, properties and applications of solid catalysts prepared by solution combustion synthesis (SCS). Appl Catal A Gen 452:117–131. https://doi.org/10.1016/J.APCATA.2012.11.024
Papavasiliou J, Avgouropoulos G, Ioannides T (2006) In situ combustion synthesis of structured Cu–Ce–O and Cu–Mn–O catalysts for the production and purification of hydrogen. Appl Catal B Environ 66:168–174. https://doi.org/10.1016/J.APCATB.2006.03.011
Ertl G, Knoezinger H, Schueth F, Weitkamp J (2008) Handbook of heterogeneous catalysis. Wiley, Weinheim
Guo X, Zhou R (2016) A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal Sci Technol 6:3862–3871. https://doi.org/10.1039/c5cy01816a
Avgouropoulos G, Ioannides T (2003) Selective CO oxidation over CuO–CeO2 catalysts prepared via the urea–nitrate combustion method. Appl Catal A Gen 244:155–167. https://doi.org/10.1016/S0926-860X(02)00558-6
Kapteijn F, Singoredjo L, Andreini A, Moulijn JA (1994) Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl Catal B Environ 3:173–189. https://doi.org/10.1016/0926-3373(93)E0034-9
Li Y, Fu Q, Flytzani-Stephanopoulos M (2000) Low-temperature water-gas shift reaction over Cu- and Ni-loaded cerium oxide catalysts. Appl Catal B Environ 27:179–191. https://doi.org/10.1016/S0926-3373(00)00147-8
Wang H, Lu Y, Han YX et al (2017) Enhanced catalytic toluene oxidation by interaction between copper oxide and manganese oxide in Cu–O–Mn/γ-Al2O3 catalysts. Appl Surf Sci 420:260–266. https://doi.org/10.1016/j.apsusc.2017.05.133
Wöllner A, Lange F, Schmelz H, Knözinger H (1993) Characterization of mixed copper-manganese oxides supported on titania catalysts for selective oxidation of ammonia. Appl Catal A Gen 94:181–203. https://doi.org/10.1016/0926-860X(93)85007-C
Palmisano P, Russo N, Fino P et al (2006) High catalytic activity of SCS-synthesized ceria towards diesel soot combustion. Appl Catal B Environ 69:85–92. https://doi.org/10.1016/J.APCATB.2006.06.002
Imamura S, Shono M, Okamoto N et al (1996) Effect of cerium on the mobility of oxygen on manganese oxides. Appl Catal A Gen 142:279–288. https://doi.org/10.1016/0926-860X(96)00095-6
Liang Q, Wu X, Weng D, Xu H (2008) Oxygen activation on Cu/Mn-Ce mixed oxides and the role in diesel soot oxidation. Catal Today 139:113–118. https://doi.org/10.1016/j.cattod.2008.08.013
Platau A, Johansson LI, Hagström AL et al (1977) Oxidation of cerium and titanium studied by photoelectron spectroscopy. Surf Sci 63:153–161. https://doi.org/10.1016/0039-6028(77)90334-X
Biesinger MC, Payne BP, Grosvenor AP et al (2011) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci 257:2717–2730. https://doi.org/10.1016/j.apsusc.2010.10.051
Biesinger MC, Lau LWM, Gerson AR, Smart RSC (2010) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl Surf Sci 257:887–898. https://doi.org/10.1016/j.apsusc.2010.10.051
Chen D, He D, Lu J et al (2017) Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl Catal B Environ 218:249–259. https://doi.org/10.1016/j.apcatb.2017.06.053
Dupin J-C, Gonbeau D, Vinatier P, Levasseur A (2000) Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys Chem Chem Phys 2:1319–1324. https://doi.org/10.1039/A908800H
Deng W, Dai Q, Lao Y et al (2015) Low temperature catalytic combustion of 1,2-dichlorobenzene over CeO2–TiO2 mixed oxide catalysts. Appl Catal B Environ 181:848–861. https://doi.org/10.1016/j.apcatb.2015.07.053
Lin X, Li S, He H et al (2018) Evolution of oxygen vacancies in MnOx–CeO2 mixed oxides for soot oxidation. Appl Catal B Environ 223:91–102. https://doi.org/10.1016/j.apcatb.2017.06.071
Mullins DR (2015) The surface chemistry of cerium oxide. Surf Sci Rep 70:42–85. https://doi.org/10.1016/j.surfrep.2014.12.001
Machida M, Uto M, Kurogi D, Kijima T (2000) MnO(x)–CeO2 binary oxides for catalytic NO(x) sorption at low temperatures. Sorptive removal of NO(x). Chem Mater 12:3158–3164. https://doi.org/10.1021/cm000207r
Dosa M, Piumetti M, Bensaid S et al (2018) Novel Mn–Cu-containing CeO2 nanopolyhedra for the oxidation of CO and diesel soot: effect of dopants on the nanostructure and catalytic activity. Catal Lett 148:298–311. https://doi.org/10.1007/s10562-017-2226-y
Kundakovic L, Flytzani-Stephanopoulos M (1998) Reduction characteristics of copper oxide in cerium and zirconium oxide systems. Appl Catal A Gen 171:13–29. https://doi.org/10.1016/S0926-860X(98)00056-8
Liu W, Flytzani-Stephanopoulos M (1995) Total oxidation of carbon monoxide and methane over transition metal-fluorite oxide composite catalysts. J Catal 153:317–332
Reitz JB, Solomon EI (1998) Propylene oxidation on copper oxide surfaces: Electronic and geometric contributions to reactivity and selectivity. J Am Chem Soc 120:11467–11478. https://doi.org/10.1021/ja981579s
Wood BJ, Wise H, Yolles RS (1969) Selectivity and stoichiometry of copper oxide in propylene oxidation. J Catal 15:355–362. https://doi.org/10.1016/0021-9517(69)90304-2
Santos VP, Pereira MFR, Órfão JJM, Figueiredo JL (2009) Synthesis and characterization of manganese oxide catalysts for the total oxidation of ethyl acetate. Top Catal 52:470–481. https://doi.org/10.1007/s11244-009-9187-3
Li C, Xin Q, Guo X (1992) Surface oxygen species and their reactivities in the mild oxidation of ethylene on cerium oxide studied by FT-IR spectroscopy. Catal Lett 12:297–305. https://doi.org/10.1007/BF00767212
Njagi EC, Genuino HC, King’Ondu CK et al (2012) Catalytic oxidation of ethylene at low temperatures using porous copper manganese oxides. Appl Catal A Gen 421–422:154–160. https://doi.org/10.1016/j.apcata.2012.02.011
Zhang X, Wu D (2016) Ceramic monolith supported Mn–Ce–M ternary mixed-oxide (M = Cu, Ni or Co) catalyst for VOCs catalytic oxidation. Ceram Int 42:16563–16570. https://doi.org/10.1016/J.CERAMINT.2016.07.076
Morales MR, Yeste MP, Vidal H et al (2017) Insights on the combustion mechanism of ethanol and n-hexane in honeycomb monolithic type catalysts: Influence of the amount and nature of Mn–Cu mixed oxide. Fuel 208:637–646. https://doi.org/10.1016/J.FUEL.2017.07.069
Acknowledgements
This research activity has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 768692.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised with the author(s)’ decision to opt for Open Choice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marin Figueredo, M.J., Andana, T., Bensaid, S. et al. Cerium–Copper–Manganese Oxides Synthesized via Solution Combustion Synthesis (SCS) for Total Oxidation of VOCs. Catal Lett 150, 1821–1840 (2020). https://doi.org/10.1007/s10562-019-03094-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-03094-x