Abstract
This paper try to answer the question, if the exfoliated graphite/nickel/palladium (EG/Ni/Pd) composite can be regarded as a potential anode material in direct methanol fuel cells (DMFCs). For this purpose, process of electrochemical oxidation of methanol on electrode made of EG/Ni/Pd was investigated. Electrochemical investigations were conducted in alkaline medium by cyclic voltammetry and potentiostatic techniques. Informations on catalytic activity of the examined composite were acquired from the comparison of current charges calculated for methanol oxidation peaks. The investigations revealed that electrochemical activity of EG/Ni/Pd composite enhances along the potential cycling. Current charges of methanol oxidation peaks increase with number of conducted cycles up to 26th cycle. The increment in electrochemical activity impacts on the decline in methanol concentration in the investigated electrolyte. The results of electrochemical investigations for EG/Ni/Pd composite were compared to that acquired for EG/Pd. The difference in total organic carbon (TOC) before and after the selected cycles of electrochemical process at EG/Ni/Pd electrode were the source of information on degree of methanol oxidation. Process comprised of 50 cycles of methanol electrooxidation resulted over 50% decrease in its concentration. The interpretation of electrochemical investigations were supported by scanning electron microscopy (SEM), energy dispersive spectrometry (EDS) and X-ray photoelectron spectroscopy (XPS) analysis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development of science associated with the electrochemical generation of energy also concerns investigations of fuel cells [1, 2]. Direct Methanol Fuel Cells (DMFCs) are recognized as the most promising sources of energy due to application of fuel that is characterized by relatively high energy density, low costs, abundance of production, not complicated storage and ability to degradation to CO2 [3, 4]. On the other hand, the necessity of catalyst application appears to be the main disadvantage of the mentioned type of fuel cells. Among the catalyst used for oxidation of alcohols is Pt. The difficulty is that Pt is expensive, not stabile and susceptible to poisoning [4,5,6,7,8]. A variety of methods have been proposed to solve this problem. One of them consists in limitation of applied catalyst whereas another one relates with the application of selected additives. In order to improve the electrocatalytical properties, other noble metals like Ru, Pd and Au are introduced into the Pt catalyst [4, 5, 9, 10]. The improvement of electrical properties with simultaneous reduction of catalyst costs were reached due to incorporation of less expensive metals such as Ni, Fe, Co, Sn or oxides [4, 7, 8, 11, 12]. According to another solution, Pt catalyst is entirely replaced by another one. Besides the Pt, the usually used component of anode material for electrochemical oxidation of methanol is Pd [13,14,15,16]. In some aspects Pd catalyst is recognize to be more suitable for methanol oxidation in alkaline medium than Pt. This is mainly by improved methanol dehydrogenation, higher electrochemical activity, better resistivity to CO poisoning and reduction of costs [14, 15]. Taking into account the economical as well as ecological point of view, the strategy of fuel cell should be based on the reduction of metal amount used for anode preparation [7, 9, 12, 17,18,19]. Hence, in some cases catalysts used for alcohol oxidation constitute metal combination with carbon materials. Owing to their developed specific surface area, appearance of functional groups, high conductivity, carbon materials are widely applied as adsorbents [20] and/or matrix for catalyst [8, 19]. Carbon materials are also used as electrodes for alcohols oxidation [7, 9, 17,18,19, 21]. Metal catalyst are merge with carbons by variety of methods, a.g. metal dispersion on carbon materials [8] and direct deposition [18, 19]. High stability with simultaneous high electrochemical activity are provided by application of graphite intercalation compounds (GIC). They are prepared by insertion of ions and/or molecules, e.g., alkali metals, metal oxides or transition metal chlorides between the interlayer space of graphite structure [22,23,24,25]. Chemical vapor deposition (CVD) may be considered as an alternative way for synthesis of metal–carbon composites [26, 27]. In this method carbon containing hydrocarbons (a.g. acetylene) are subjected to thermal treatment over the metal catalyst yielding different forms of carbon.
Our previous works showed that it is possible to prepare a stable, electrochemically active composite material on the basis of exfoliated graphite by electrodeposition of selected metals on its surface [28, 29]. Thus synthetized composite materials exhibit properties that enable their application as active electrodes for organic compounds oxidation as well as active materials for electrochemical sorption of hydrogen.
Our work reported that exfoliated graphite/nickel/palladium (EG/Ni/Pd) composite exhibits high electrochemical activity toward the processes of reversible electrosorption of hydrogen [30]. Electrochemical measurements comprising of cyclic potential scanning interrupted by the potentiostatic saturation of composite electrode with hydrogen provided an important information on the mechanism of hydrogen accumulation within the respective parts of the examined composite. The described researches clearly showed that capability for hydrogen sorption of EG/Ni/Pd electrodes results from the electrochemical activity of all its components.
The goal of the present work was to study the process of methanol electrooxidation over the EG/Ni/Pd electrode. To evolve electrocatalytical properties of the mentioned composite, cyclic voltammetry and potentiostatic investigations were performed. Electrochemical activity of the EG/Ni/Pd was correlated with that acquired for EG/Pd composite. The efficiency of methanol electrooxidation on EG/Ni/Pd electrode was discussed in relation to changes in methanol concentration. The indirect information on methanol concentration were gathered from the TOC analysis of the investigated electrolyte. By comparing the SEM images and EDS calculations of electrode content before and after the electrochemical process, some conclusions on material stability were drawn.
2 Experimental
2.1 Electrode Preparation
Process of EG/Ni/Pd formation comprised of three independent steps. Within the former one, exfoliated graphite (EG) was prepared by thermal exfoliation of graphite intercalation compounds with sulphuric acid (H2SO4-GIC). Electrodeposition of Ni over the previously obtain EG yielded EG/Ni. The above mentioned process was performed by galvanostatic mode in NiSO4·7H2O, NiCl2·6H2O and H3BO3 using a constant current density of 20 mA g−1. During the last step, owing to 3 min lasting chemical reaction of EG/Ni with PdCl2/HCl water solution, a final product EG/Ni/Pd was synthetized [30].
2.2 Electrochemical Oxidation of Methanol
All electrochemical investigations were realized in three-electrode system in electrolyte composed of 0.1 M methanol diluted in 6 M KOH solution with its permanent stirring. Electrochemical oxidation of methanol were realized by cyclic voltammetry (CV) and potentiostatic techniques. Powder type EG/Ni/Pd electrode served as a working electrode, Hg/HgO/6 M KOH electrode was used as a reference electrode (0.098 V vs. NHE), whereas graphite rod (5 mm in diameter) was playing a role of counter electrode. The mass of working electrode was equal to 25 mg. More details of electrode preparation can be found in our previous papers [22,23,24,25, 30, 31]. Voltammetric measurements were performed with a scan rate 10 mV s−1 within the potential range of − 0.7 ↔ 0.3 V, starting from the rest potential of electrode (ER) towards the more negative potentials. Potentiostatic measurements were realized under constant potential corresponding to the maxima of methanol oxidation peaks recorded on cyclic voltammograms. To understand the origin of EG/Ni/Pd activity, electrochemical investigations for EG/Pd composite were done under conditions elsewhere described for EG/Ni/Pd composite. EG/Pd was formed by electrodeposition of Pd onto the EG surface from the bath composed of PdCl2/HCl water solution. Process was conducted by galvanostatic route under constant current density of 100 mA g−1 for 4 h.
All electrochemical investigations were performed on Autolab PGSTAT 302 N potentiostat/galvanostat.
2.3 Composite Characterization
Morphology of EG/Ni/Pd and EG/Pd composites were examined by scanning electron microscopy (SEM) (S-3400 N, Hitachi microscope) using a voltage acceleration of electron beam equal to 15 keV. Changes in chemical composition of EG/Ni/Pd surface caused by the process of methanol electrooxidation were estimated from the EDS analysis (Energy Dispersive Spectrometry).
In order to examine the oxidation stage of Ni and Pd before and after 50 cycles of methanol electrooxidation the XPS analysis were carried out. The measurements were performed in a PHI VersaProbeII Scanning XPS system using monochromatic Al Kα (1486.6 eV) X-rays focused to a 100 µm spot and scanned over the area of 400 µm × 400 µm.
The efficiency of methanol electrooxidation over the examined EG/Ni/Pd was calculated on the basis of total organic carbon (TOC) measurements. The difference in TOC between the starting electrolyte and after the respective time of methanol oxidation indicated the amount of organic carbon underwent transformation. It is assumed that the main product of methanol electrooxidation appears to be CO2, however it cannot be excluded that some amount of by-products such as carbonates are also present in the electrolyte after the process. To explain how the degree of mentioned degradation changes along the potential scanning, during the selected cycles (1, 5, 10, 25 and 50 cycle) at the potential related to ER, the samples of electrolyte were taken out from the electrolytic cell for TOC analysis. The TOC examinations were performed on TOC-L analyser (Shimadzu).
3 Results and Discussion
3.1 Electrochemical Investigations of EG/Ni/Pd Composite in Electrolyte Free of Methanol
The investigated EG/Ni/Pd composite was widely described in our previous work [30]. On the basis of XRD analysis two essential conclusions have been stated. The former one that all components of EG/Ni/Pd coexists as a separate phases while the latter statement is that there is no Ni-Pd alloy within the regarded composite. Moreover, the EDS analysis have revealed that surface of the investigated composite is characterized by average Pd/Ni ratio of 7:1. It is worth to note that besides the metal contents, EG/Ni/Pd surface is composed of C and O atoms (81 and 11%, respectively).
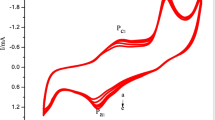
In Fig. 1 are shown voltammetric cycles recorded for EG/Ni/Pd composite in 6 M KOH in the potential range of − 0.7 ↔ 0.3 V with scan rate of 10 mV s−1. After starting the measurement one can observe a cathodic peak at the potential of − 0.239 V. This peak is likely associated with the reduction reaction of Pd oxides originally present on electrode surface. On further cycling (cycles 2 and 3) a significant increase in intensity of cathodic peak accompanied by its shift toward the less negative potentials (− 0.197 V) is noted. Such a behavior indicates that during the second and third cycle the regarded cathodic peaks are likely associated with reduction of Pd oxides formed on the surface of electrode during the previously performed anodic oxidation [15, 19]. For the first cycle, during the anodic polarization, presented curve involves anodic wave with two maxima positioned at − 0.602 and − 0.49 V. These effects can be assigned to oxidation of Ni particles [7, 15, 22,23,24,25, 30]. During the second and third cycle the location of these peaks vary due to activation of Ni. On further anodic scanning up to 0.3 V, oxidation of EG/Ni/Pd surface occurs.
3.2 Voltammetric Oxidation of Methanol Over the EG/Ni/Pd Composite
Figure 2 displays three cycles of voltammetric oxidation of 0.1 M CH3OH diluted in 6 M KOH at electrode made of EG/Ni/Pd composite. As can be seen, during the initial cycles the investigated composite did not exhibit a significant activity toward the process of methanol oxidation. The mentioned reaction is depicted as a wide anodic wave spreading out from − 0.3 to 0.1 V. The observed wave involves two maxima: at − 0.19 (peak A1) and 0.026 V (peak A3). During the first cycle, the latter effect is dominant while for the subsequent cycles, peak A1 gradually grows up becoming more and more intensive. The regarded wave likely illustrates reaction of methanol oxidation (peak A1) and oxidation of EG/Ni/Pd surface (peak A3) [15, 16, 19]. On the cathodic side one can observe two coupled effects. At the potential of − 0.145 V emerges cathodic peak (peak C) followed by anodic one positioned at − 0.247 V (peak A2). The former peak corresponds to reduction of previously formed Pd oxides, while the latter effect depicts removal of carbonaceous species originating from methanol oxidation not entirely oxidized during the forward scanning with simultaneous reaction of methanol oxidation on freshly prepared electrode surface [15, 16, 19]. For the 2 and 3 cycle peak A2 gradually increases and shifts toward the less negative potentials.
It is assumed that the starting cycles of regarded process can be recognized as an activating cycles. On further cycling, the shape of presented CVs become more typical for the electrochemical oxidation of methanol over the Pd catalyst.
Figure 3 displays voltammograms recorded during 25 cycles of methanol oxidation on EG/Ni/Pd composite. As can be seen, the consecutive cycles indicate gradual increment in electrochemical activity of EG/Ni/Pd composite. The intensity as well current charges of both anodic peaks associated with methanol oxidation (A1 and A2) steeply grow up along the cycling. However, it should be noted that depending on the regarded cycle, the degree of current increment differs. In case of anodic peak recorded during the forward scanning (peak A1) the most pronounced increase in intensity is observed up to 16 cycle, after that only slight rise in currents is noted. For this peak the upward trend is preserved up to the 25 cycle. Simultaneously, the intensity as well as current charges of peak A2 consecutively increase on cycling. Such a behavior indicates increasing efficiency of recovery of EG/Ni/Pd surface allowing the secondary reaction of methanol oxidation to carry out. The particular observation of peak A1 enables to find some asymmetry on its right shoulder (− 0.075 V). The presence of pre-peak A *1 indicates that the process of methanol oxidation illustrated by the mentioned peak has a multistep character.
To acquire an information on long lasting activity of EG/Ni/Pd composite in the process of methanol electrooxidation, the number of potential scanning was extended to 50 cycles.
Figure 4 shows CVs for EG/Ni/Pd recorded during cycles 26–50 in 0.1 M CH3OH in 6 M KOH with scan rate of 10 mV s−1. Taking into account the composite activity towards the methanol oxidation, the crucial step seems to occur in 27 cycle. During this cycle, the intensity as well as current charge of peak associated with the primary reaction of methanol oxidation (peak A1) slightly decrease. For 26th cycle charge density of peak A1 reached 55.6 C g−1, whereas for 27th cycle 48.4 C g−1. Upon further cycling the regarded peak is partially stabilized, only slight decrease in recorded currents is noted. It is worth to mention that it still has a asymmetric shape suggesting the multistep character of the conducted process. Similar to peak A1, anodic peak associated with the removal of intermediate products and secondary reaction of methanol oxidation (peak A2) also exhibit slight downward tendency after the 26th cycle. Charge density of this peak decreases from 18.3 C g−1 for 26th cycle to 17.3 C g−1 for 27th cycle. It should be also mentioned that the reactions associated with the anodic oxidation of Pd to Pd oxides are steeply extinguished with cycling. After the 26th cycle anodic effects spreading on the potential range from 0 to 0.3 V become negligible small.
3.3 Voltammetric Oxidation of Methanol Over the EG/Pd Composite
To understand the origin of EG/Ni/Pd activity, electrochemical measurements for EG/Pd composite were done under conditions described for EG/Ni/Pd composite. In Figs. 5 and 6 are shown cyclic voltammograms for EG/Pd composite in 0.1 M CH3OH diluted in 6 M KOH recorded during 1–25 and 26–50 cycles, respectively. Similar to EG/Ni/Pd composite (Figs. 3 and 4), the presented curves involve anodic peaks corresponded to methanol oxidation. However, the location as well as intensity of the considered peaks significantly differ compared to that observed for EG/Ni/Pd. Signals for two-component composite (EG/Pd) are markedly less intensive indicating its lower electrochemical activity. This statement is supported by the values of density charges of methanol oxidation peaks. For EG/Pd the calculated densities are several dozen times lower than that for EG/Ni/Pd. Upon cycling this discrepancy lowers but it is still significant. After 50th cycle, the charge density of methanol oxidation peaks for EG/Pd is equal to 7.58 C g−1 whereas for EG/Ni/Pd 60.4 C g−1. The acquired results justify the statement that the higher electrocatalytical effect is reached when the reaction of methanol oxidation proceeds on the Pd/Ni interface. It is worth to note that in case of EG/Pd (Figs. 5 and 6) the maxima of methanol oxidation peaks are shifted toward the less negative potentials as compared to the EG/Ni/Pd composite (Figs. 3 and 4). By comparing the location of methanol oxidation peaks shown in Fig. 3, 4, 5 and 6, it is plausible that for EG/Ni/Pd an anodic effect denoted as A *1 arise from the reaction of methanol electrooxidation occurring on Pd. Taking into account fact, that Ni do not exhibits increased activity towards the methanol electrooxidation within the applied potential range, the huge anodic peaks shown on CVs for EG/Ni/Pd (Fig. 3, 4) mostly arises from the Pd component. However it cannot be excluded that Ni particles undergo oxidation yielding Ni(OH)2 which can be useful for oxidation of intermediate products of methanol oxidation.
3.4 Potentiostatic Oxidation of Methanol
In Fig. 7 are presented potentiostatic curves for EG/Ni/Pd and EG/Pd composites recorded during electrooxidation of 0.1 M methanol in 6 M KOH. In order to differentiate the electrochemical activity of the respective components of EG/Ni/Pd composite, the presented plot also involves a curve for EG/Ni. Potentiostatic measurements were conducted under constant potential corresponding to the potentials of maxima of methanol oxidation peaks provided from the forward scanning on cyclic voltammograms (Figs. 3, 4, 5 and 6). During the initial step, a rapid falls down of current densities caused by a rapid polarization of EG/Ni/Pd and EG/Pd electrodes is observed. After that the process is stabilized and proceeds through the long lasting step depicted as current plateau. Comparison of anodic charges undoubtedly confirmed that the EG/Ni/Pd composite is significantly more active towards the electrochemical oxidation of methanol than EG/Pd as well as EG/Ni. Charge density calculated for three component composite after 1 h anodic oxidation is over 24 time higher (0.314 A g−1) as compared to that noted for EG/Pd (0.018 A g−1). Plateau for EG/Ni/Pd has irregular character likely indicating that the process proceeds with accompaniment of gases evolution, probably CO2. It is worth to note that results of potentiostatic investigations coincide with the results acquired by voltammetric technique.
On the basis of obtained results it can be stated that among the investigated samples EG/Ni/Pd composite displays highest electrochemical activity toward the process of methanol oxidation. High stability of the electrochemical activity during long lasting process may be recognized as a desired property allowing to consider the investigated composite as a potential anode in DMFC. However, to support the above mentioned thesis, the amount of oxidized methanol should be detected.
3.5 Total Organic Carbon Measurements
In order to determine the decline in methanol concentration caused by its cyclic electrooxidation on EG/Ni/Pd composite, the measurements of total organic carbon (TOC) were performed. Taking into account the mechanism of methanol electrooxidation it was assumed that depletion of TOC will be related with the decrease in methanol concentration due to its oxidative transformation into the inorganic carbon mainly represented by CO2 but also carbonates. For this purpose after the selected cycles of methanol oxidation (1, 5, 10, 25 and 50 cycle), the samples of electrolyte were taken out from the electrolytic cell to perform TOC analysis. Data of TOC analysis correlated with charge density of methanol oxidation peaks are included in Table 1, whereas the graphical illustration of the relationship between TOC results and total charge consumed for oxidation of methanol after the respective cycles are shown in Fig. 8. Basing on the above mentioned assumption, it can be stated that after the 50 cycles of electrochemical oxidation, methanol concentration in electrolyte is reduced of over 50%. In other words, owing to the electrochemical activity of EG/Ni/Pd electrode over 50% of methanol is transformed into the inorganic carbon due to 50 cycles lasting process. Taking into account the fact that in some part the exhibited voltammograms have a frayed character indicating gas evolution it cannot be excluded that the main product of the conducted process is CO2. The decline in methanol concentration noted along the cycling is not linear. The rate of methanol oxidation accompanied by the formation of inorganic carbon seems to be strongly correlated with the electrochemical activation of EG/Ni/Pd electrode recognized as a charge density of methanol oxidation peaks. When the upward tendency of current charges depicting an increase in electrode activity is observed (25 cycles of conducted process) (Fig. 3) over 32% of methanol become transformed into the inorganic carbon. On further oxidation up to 50th cycle methanol concentration is reduces of only 17.8%. This behavior coincides with the downward tendency of peak charges of methanol oxidation (A1 and A2) noted after the 26 cycle (Fig. 4). As seen from Fig. 8, the total charge density of 3046 C g−1 (calculated for both anodic peaks for all 50 cycles) was consumed for over 50% oxidation of methanol to inorganic carbon (line a). On regarding the investigated EG/Ni/Pd as a potential anode for DMFC the above mentioned relation can be expressed that the 50% oxidation of methanol fuel could generate the amount of energy which corresponds to 3046 C g−1 (line b). To acquire the total charge density of almost 150 C g−1 over 32% of methanol should be oxidized to inorganic carbon. For this electrode it is reached after 25 cycles. It means that during the potential cycling up to 25th cycle relatively more energy is generated compared to the process conducted after 25th cycle. This result agrees with the statement that after 26th cycle when the activity of electrode slightly decreases, the efficiency of methanol transformation into the inorganic product is lower compared to that observed during the previous 25 cycles. The gentle deactivation of EG/Ni/Pd electrode probably is associated with its poisoning effect caused by deposited CO [5, 8, 10, 17,18,19]. It seems that the mechanism of electrode poisoning can be similar to that given by Zhu et al. [32]. On the other hand it cannot be excluded that decrease in intensity of methanol oxidation peak (A1 in Fig. 4) after reaching the 26 cycle can be caused by the undesired impact of alkaline solution. It is known that in some cases the concentrated electrolyte involving even trace impurities may have unfavorable effect on the electrode behavior [33, 34]. Owing to the considerable higher activity of Pd it can be assumed that the unwanted problem of electrode poisoning by CO is mainly associated with Pd component of the investigated system.
It is worth to mention that low decline in electrode activity indicates its high resistivity against poisoning. To explain the reasons for a such behavior the changes in morphology of electrode material caused by electrochemical oxidation of methanol should be examined. For this purpose SEM analysis of EG/Ni/Pd composite before and after the 50 cycles of methanol oxidation was performed.
3.6 Analysis of morphology and distribution of components within the EG/Ni/Pd composite
Figure 9 presents SEM images recorded for EG/Ni/Pd electrode before (Fig. 9a, b) and after 50 cycles (Fig. 9c, d) of methanol oxidation. To emphasize the differences in surface microstructure of the investigated composite additional SEM image depicting EG/Pd (e) was added to Fig. 9. The shape as well as size of Pd particles existing on EG/Ni/Pd differ significantly compared to Ni particles. Pd particles have a flower like structure and are markedly bigger. Comparison of SEM images of EG/Ni/Pd composite revealed that its morphology changes due to long lasting electrochemical oxidation of methanol. The surface of spherical structures representing Pd after the electrochemical measurements become irregular (see Fig. 9c and d). It seems that some Pd particles are coated with bright elements characterized by poor electrical conductivity probably arise from the CO species being the products of methanol oxidation. On the other hand, the irregular shape of Pd particles and some sharp edges seen in Fig. 9c may suggest that formation of surface defects accompanied by the partial agglomeration of Pd particles occur. The EDS analysis revealed some changes in average concentration of composite components caused by 50 cycles of voltammetric oxidation of methanol. The results of EDS analysis are given in Table 2. It is seen that due to electrochemical treatment of EG/Ni/Pd in methanol containing 6 M KOH increases concentration of C and O atoms. In parallel, amount of Pd significantly fell down in consequence of performed electrooxidation. Such a behavior evidences partial poisoning of electrode surface by deposited CO being the intermediate product of methanol electrooxidation.
The distribution of metal components within the EG/Ni/Pd composite can be examined by EDS mapping. The resulted mapping (not presented here) have showed that however the Ni and Pd are relatively well dispersed over the surface of EG, but particular observation evidences that the edges and surface defects are favorable to metal deposition, hence increased concentration of metal deposits can be seen in these parts of composite surface. Moreover it should be noted that the presented mapping did no show significant changes in metal distribution over the electrode surface due to the process of methanol electrooxidation.
XPS analysis of Ni and Pd regions (Figs. 10 and 11, respectively) evidences that Ni component of the examined composite mainly exists as Ni2+. Taking onto account the location of main peaks as well as an environmental used to conduct process it is plausible that Ni appears as Ni(OH)2 [35] whereas Pd occurs within the EG/Ni/Pd composite in metallic form and Pd2+ (PdO) [36]. From the comparison of the peaks in Fig. 11 it is evident that Pd0 is dominant. However the above mentioned tendency does not change due to methanol oxidation but it seems that the participation of Pd2+ slightly increases after 50 cycles of the conducted process. It can be easily explained by the formation of palladium oxide which is a side reaction of methanol oxidation. On the other hand, the appearance of PdO within the starting material can be associated with the partial oxidation of EG/Ni/Pd surface in air. Such a behavior well coincides with the electrochemical results presented in Fig. 1.
4 Conclusions
The presented investigations evidenced high electrochemical activity of EG/Ni/Pd composite toward the process of methanol electrooxidation. Cyclic voltammetry measurements revealed increment in electrocatalytic activity of the examined composite upon cycling. This upward tendency is observed up to 26th cycle, than activity slightly decreases. Taking into account the value of total organic carbon as a criterion of methanol oxidation to inorganic carbon (probably CO2) it can be concluded that after 50 cycles of oxidation over 50% of initial methanol is oxidized. This effect is accompanied by the generation of over 3000 C g−1 of charge density.
From the acquired results it is seen that higher rate of methanol oxidation is reached during the process occurring up to 25 cycle. During the further cycling the intensity of the conducted process slightly declines probably due to some changes in composition of EG/Ni/Pd surface and/or poisoning effect. Taking into consideration the low scale of electrode deactivation it can be stated that the investigated composite exhibits high stability and resistivity against poisoning.
The comparative study of electrochemical oxidation of methanol on EG/Ni/Pd and EG/Pd evidenced that to reach high efficiency of oxidation the binary catalyst Ni/Pd is needed.
Summarizing, it can be concluded that electrochemical activity as well as high stability justify the considering of EG/Ni/Pd composite as an anode material in DMFCs. However, before its practical application in commercial fuel cells additional investigations concerning determination of by-products produced as well as detailed characterization of composite surface should be performed.
References
Liu H, Zhang J (2009) Electrocatalysis of direct methanol fuel cells. Wiley-VCH Verlag GmbH&Co, Weinheim
Litster S, McLean G (2016) PEM fuel cell electrodes. J Power Sources 303:182–193
Lei H, Atanassova P, Sun Y, Blizanac B (2009) State-of-the-art electrocatalysis of direct methanol fuel cells. In: Liu H, Zhang J (eds) Electrocatalysis of direct methanol fuel cells. Wiley-VCH Verlag GmbH&Co, Weinheim
Wasmus S, Küver A (1999) Methanol oxidation and direct methanol fuel cells: a selective review. J Electroanal Chem 461:14–31
Burnstein GT, Barnett CJ, Kucernak AR, Wiliams KR (1997) Aspects of the anodic oxidation of methanol. Catal Today 38:425–437
Yu EH, Scott K, Reeve RW (2003) A study of the asnodic oxidastion of methanol on Pt in alkline solutions. J Electroanal Chem 547:17–24
Cui X, Cui F, He Q, Guo L, Ruan M, Shi J (2010) Graphitized mesoporous carbon supported Pt-SnO2 nanoparticles as a catalyst for methanol oxidation. Fuel 89:372–377
Rodriguez JR, Fuentes-Moyado S, Zepeda TA, Díaz de León JN, Cruz-Reyes J, Oropeza-Guzman MT, Berhault G, Alonso-Núñez G (2016) Methanol electro-oxidation with alloy nanoparticles of Pt10−x–Fex supported on CNTs. Fuel 182:1–7
Biswas PC, Nodasaka Y, Enyo M, Haruta M (1995) Electro-oxidation of CO and methanol on graphite-based platinum electrodes combined with oxide-supported ultrafine gold particles. J Electroanal Chem 381:167–177
Jeon MK, Cooper JS, McGinn PJ (2008) Methanol electro-oxidation by a ternary Pt–Ru–Cu catalyst identified by a combinatorial approach. J Power Sources 185:913–916
Stalnionis G, Tamašauskaite-Tamašiūnaite L, Pautieniene V, Jusys Z (2004) Modification of a Pt Surface by spontaneous Sn deposition for electrocataltyic applications. Oxidation of CO, formaldehyde, formic acid, and methanol. J Solid State Electrochem 8:900–907
Sun J, Dou M, Zhang Z, Wang JJF (2016) Carbon nanotubes supported Pt-Co-P ultrafine nanoparticle electrocatalysts with superior activity and stability for methanol electro-oxidation. Electrochim Acta 215:447–454
Singh RN, Singh Anindita A (2009) Electrocatalytic activities of binary and ternary composite electrodes of Pd, nanocarbon and Ni for electro-oxidation of methanol in alkaline medium. J Solid State Electrochem 13:1259–1265
Antolini E, Gonzalez ER (2010) Alkaline direct alcohol fuel cells. J Power Sources 195:3431–3450
Song Y, Sen X, Yang S, Wei X, Sun Z (2016) Electrocatalytic performance for methanol oxidation on nanoporous Pd/NiO composites prepared by one-step dealloying. Fuel 181:269–279
Jurzinsky T, Kammerer P, Cremers C, Pinkwart K, Tübke J (2016) Investigation of ruthenium promoted palladium catalysts for methanol electrooxidation in alkaline media. J Power Sources 303:182–193
Gloaguen F, Léger JM, Lamy C (1997) Electrocatalytic oxidation of methanol on Platinum nanaoparticles electrodeposited onto porous carbon substrates. J Appl Electrochem 27:1052–1060
Park S, Xie Y, Weaver M (2002) Electroacatalytic pathways on carbon-supported platinum nanoparticles:comparison of particle-size-dependent rates of methanol, formic acid, and formaldehyde electrooxidation. Langmuir 18:5792–5798
Shen PK, Xu C, Zeng R, Liu Y (2006) Electro-oxidation of methanol on Pt/C and Pd/C catalyst. Electrochem Solid St 9:A39–A42
Gómez-Serrano V, Álvarez PM, Jaramillo J, Beltrán FJ (2002) Formation of oxygen complexes by ozonation of carbonaceous materials prepared from cherry stones I. Thermal effects. Carbon 523:513–522
Comignani V, Sieben JM, Brigante ME, Duarte MME (2015) Carbon supported Pt-NiO nanoparticles for ethanol electro-oxidation in acid media. J Power Sources 278:119–127
Skowroński JM, Krawczyk P, Rozmanowski T, Urbaniak J (2008) Electrochemical behavior of exfoliated NiCl2-graphite intercalation compound affected by hydrogen sorption. Energy Convers Manag 49:2440–2446
Skowroński JM, Rozmanowski T, Krawczyk P (2013) Enhancement of electrochemical hydrogen storage in NiCl2–FeCl3–PdCl2–graphite intercalation compound effected by chemical exfoliation. Appl Surf Sci 275:282–288
Skowroński JM, Czerwiński A, Rozmanowski T, Rogulski Z, Krawczyk P (2009) The investigation on the mechanism of electrochemical hydrogen storage in sandwich nickel foam/palladium/carbon nanofibers electrodes. J Nanosci Nanotechnol 9:3858–3865
Rozmanowski T, Krawczyk P (2016) Changes in structure, morphology and electrochemical properties of NiCl2–FeCl3–PdCl2–graphite intercalation compound affected by gaseous hydrogen reduction process. Electrochim Acta 205:266–272
Randall L, Wal V, Hall LJ (2003) Nanotube synthesis upon stainless steel meshes. Carbon 41:659–672
Zhao J, Guo Q, Shi J, Liu L, Jia J, Liu Y, Wang H (2009) Carbon nanotube growth in the pores of expanded graphite by chemical vapor deposition. Carbon 47:1747–1751
Krawczyk P (2013) Properties of an EG/Fe/C composite modified by ozone treatment. Carbon 65:374–376
Krawczyk P, Rozmanowski T (2015) Preparation and electrochemical properties of EG/Fe2O3/C composite. Ionics 21:59–66
Krawczyk P, Rozmanowski T, Osińska M (2016) Electrochemical sorption of hydrogen in exfoliated graphite/nickel/palladium composite. Int J Hydrogen Energy 41:20433–20438
Krawczyk P, Skowroński JM (2012) Electrochemical reactivation of expanded graphite electrodes covered by oligomeric products of phenol electrooxidation. Electrochim Acta 79:202–209
Zhu F, Ma G, Bai Z, Hang R, Tang B, Zhang Z, Wang X (2013) High activity of carbon nanotubes supported binary and ternary Pd-based catalyst for methanol, ethanol and formic acid electro-oxidation. J Power Sources 242:610–620
Martin MH, Lasia A (2009) Hydrogen sorption in Pd monolayers in alkaline solution. Electrochim Acta 54:5292–5299
Hubkowska K, Soszko M, Symonowicz M, Łukaszewski M, Czerwiński A (2017) Electrochemical behavior of a Pd thin film electrode in concentrated alkaline media. Electrocatalysis 8:295–300
Biesinger MC, Lau LWM, Gerson AR, Smart RSC (2012) The role of the Auger parameter in XPS studies of nickel metal halides and oxides. Phys Chem Chem Phys 14:2434–2442
Brun M, Berthet A, Bertolini JC (1995) XPS, AES and Auger parameter of Pd and PdO. J Electron Spectrosc Relat Phenom 104:55–60
Acknowledgements
This work was financially supported from the grant of the Poznan University of Technology No. 03/31/DSPB/0335.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krawczyk, P., Rozmanowski, T. & Frankowski, M. Methanol Electrooxidation at Electrodes Made of Exfoliated Graphite/Nickel/Palladium Composite. Catal Lett 149, 2307–2316 (2019). https://doi.org/10.1007/s10562-019-02785-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02785-9