Abstract
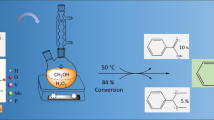
Vanillin hydrodeoxygenation was investigated using Pt/C catalyst in the temperature and total pressure ranges of 80–200 °C and 20–30 bar in several solvents, such as tetrahydrofuran, 2-propanol, water and in solventless conditions using 1:1 mass ratio of vanillin to guaiacol. The results revealed that the rate increased with increasing solvent polarity as follows: tetrahydrofuran < 2-propanol < water. The main product was p-creosol with 66% selectivity at complete vanillin conversion in HDO under 30 bar total pressure at 100 °C after 4 h using water as a solvent. In a solventless experiment with 1:1 mass ratio of vanillin–guaiacol as a feedstock only vanillin was transformed to p-creosol with 91% conversion in 4 h at 200 °C under 30 bar total pressure, while guaiacol did not produce any HDO products. Both thermodynamic analysis and kinetic modelling were performed. Vanillin hydrodeoxygenation resulted in formation of p-creosol over Pt/C catalyst using an optimum vanillin initial concentration in water solution. From the industrial point of view vanillin hydrodeoxygenation proceeded rapidly giving high yields of p-creosol in solventless hydrodeoxygenation of vanillin-guaiacol mixture, while guaiacol was not deoxygenated.
Graphical Abstract











Similar content being viewed by others
Abbreviations
- K :
-
Rate constant
- K 0 j :
-
Equilibrium constant at standard conditions for reaction j
- K eq :
-
Adsorption equilibrium constant
- N :
-
Moles (mol)
- P :
-
Pressure (bar)
- P 0 :
-
Standard pressure (bar)
- R :
-
Reaction rate
- R 2 :
-
Coefficient of determination
- R :
-
Ideal gas constant (J/K/mol)
- T :
-
Absolute temperature (K)
- T 0 :
-
Absolute standard temperature (K)
- y:
-
Yield
- ΔG 0 f :
-
Gibbs free energy of formation at standard conditions (J/mol)
- ΔG 0 r :
-
Gibbs free energy of reaction at standard conditions (J/mol)
- ΔG Φ r,j :
-
Gibbs free energy of reaction at 1 bar and chosen temperature (J/mol)
- ΔG r,j :
-
Gibbs free energy of reaction at a fixed temperature and pressure (J/mol)
- ΔH 0 f :
-
Enthalpy of formation at standard conditions (J/mol)
- ΔH 0 r :
-
Enthalpy of reaction at standard conditions (J/mol)
- ρ B :
-
Catalyst bulk density
- θ :
-
Objective function
- ν i,j :
-
Stoichiometric matrix composed by i components and j reactions (–)
References
Pandey MP, Kim CS (2011) Lignin depolymerization and conversion. Chem Eng Technol 34:29–41
Welker CM, Balasubramanian VK, Petti C, Rai KM, De Bolt S, Mendu V (2015) Engineering plant biomass lignin content and composition for biofuels and bioproducts. Energies 8:7654–7676
Sluiter JB, Ruiz RO, Scarlata CJ, Sluiter AD, Templeton DW (2010) Compositional analysis of lignocellulosic feedstocks. J Agric Food Chem 58:9043–9053
Kim DE, Pan X (2010) Preliminary study on converting hybrid poplar to high-value chemicals and lignin using organosolv ethanol process. Ind Eng Chem Res 49:12156–12163
Alriols MG, García A, Llano-Ponte R (2010) Combined organosolv and ultrafiltration lignocellulosic biorefinery process. Chem Eng J 117:113–120
Huijgen WJJ, Reith JH (2010) Pretreatment and fractionation of wheat straw by an acetone-based organosolv process. Ind Eng Chem Res 49:10132–10140
Wang H, Tucker M, Ji Y, Recent development in chemical depolymerization of lignin. Appl Chem (2013). https://doi.org/10.1155/2013/838645
Toledano A. Serrano L. Labidi J (2012) Organosolv lignin depolymerization with different base catalysts. Chem Technol Biotechnol 87:1593–1599
Hussin MH, Rahim AA, Ibrahim MNM, Brosse N (2013) Physicochemical characterization of alkaline and ethanol organosolv lignins from palm oil (Elaeis guineensis) fronds as phenol substitutes for green material applications. Ind Crops Prod 49:23–32
Oh S, Hwang H, Choi HS, Choi JW (2015) The effects of noble metal catalysts of the bio-oil quality during the hydrodeoxygenative upgrading process. Fuel 153:535–543
He L, Qin Y, Lou H. Chen P (2015) Highly dispersed molybdenum carbide nanoparticles supported on activated carbon as an efficient catalyst for the hydrodeoxygenation of vanillin. RSC Adv 5:43141–43147
Jiang L, Zhou P, Zhang Z, Jin S (2018) Nitrogen-doped carbon supported Co catalysts: an effective none-noble metal catalyst for the upgrade of biofuels. ChemSusChem. https://doi.org/10.1002/cssc.201702078
Yang H, Nie R, Wang X, Yu X, Jin D, Lu X, Zhou D (2017) Co embedded within biomass-derived mesoporous N-doped carbon as an acid-resistant and chemoselective catalyst for transfer hydrodeoxygenation of biomass with formic acid. Green Chem 19:5714–5722
Zhang X, Tang W, Zhang Q, Wang T, Ma L (2017) Hydrocarbons production from lignin-derived phenolic compounds over Ni/SiO2 catalyst. Energy Procedia 105:518–523
Zhang X, Zhang Q, Wang T, Ma T, Yu Y, Chen L (2013) Hydrodeoxygenation of lignin-derived phenolic compounds to hydrocarbons over Ni/SiO2-ZrO2 catalysts. Biores Technol 134:73–80
Nie R, Yang H, Zhang YH, Yu X, Zhou X, Zia Q (2017) Mild temperature hydrodeoxygenation of vanillin over porous nitrogen-doped carbon black supported nickel nanoparticles. Green Chem 19:3126–3134
Parsell TH, Owen BC, Klein I, Jamell TM, Marcum CL, Haupert LJ, Amundson LM, Kenttämaa HI, Ribeiro F, Miller JT, Abu-Omar MM (2013) Cleavage and hydrodeoxygenation (HDO) of C-O bonds relevant to lignin conversion using Pd/Zn synergistic catalysts. Chem Sci 4:806–813
Jiang H, Yu X, Peng X, Zhang H, Nie R, Lu X, Zhou D, Xia Q (2016) Efficient aqueous hydrodeoxygenation of vanillin over mesoporous carbon-nitride modified Pd nanoparticles. RSC Adv 6:69045–69051
Zhu Z, Tan H, Wang J, Yu S, Zhou K (2014) Hydrodeoxygenation of vanillin as a bio-oil model over carbonaceous microspheres-supported Pd catalysts in the aqueous phase and Pickering emulsions. Green Chem 16:2636–2643
Lv Z, Meng X, Xiao F-S (2013) Superhydrophilic mesoporous sulfonated melamine-formaldehyde resin supported palladium nanoparticles as an efficient catalyst for biofuel upgrade. J Mat Chem A 1:8630–8635
Bindwal AB, Vaidya PD (2014) Reaction kinetics of vanillin hydrogenation in aqueous solutions using a Ru/C catalyst. Energy Fuels 28:3357–3362
Yang XM, Liang YY, Cheng YY, Song W, Wang XF, Wang ZC, Qiu JS (2014) Hydrodeoxygenation of vanillin over carbon nanotube-supported Ru catalysts assembled at the interface of emulsion droplets. Catal Commun 47:28–31
Yang XM, Liang Y, Zhao X, Song YF, Hu LH, Wang ZC, Qiu JS (2014) Au/CNTs catalyst fir highly selective hydrodeoxygenation of vanillin at the water/oil interface. RSC Adv 4:31932–31936
Zemansky MW, Abbott MM, Van Ness HC (1975) Basic engineering thermodynamics. McGraw-Hill, New York
Mebane RC, Mansfield AJ (2005) Transfer hydrogenation of aldehydes with 2-propanol and Raney nickel. Synth Comm 5:3083–3086
Mäki-Arvela P, Tokarev A, Murzina E, Campo B, Heikkilä T, Brozinski J, Wolf D, Murzin D (2011) Kinetics of lactose and rhamnose oxidation over supported metal catalysts. Phys Chem Chem Phys 13:9268–9280
Santos JL, Alda-Onggar M, Fedorov V, Peurla M, Eränen K, Mäki-Arvela P, Centeno MA, Murzin DY (2018) Hydrodeoxygenation of vanillin over carbon supported metal catalysts. Appl Catal A 561:137–149
Risner C, Kiser M, High-performance liquid chromatography procedure for the determination of flavor enhancers in consumer chocolate products and artificial flavors. J Sci Food Agric 88:1423–1430 (2008)
Joback KG, Reid RC (1987) Estimation of pure-component properties from group-contributions. Chem Eng Comm 57:233–243
Badawi M, Paul JF, Cristol S, Payen E, Romero Y, Richard F, Brunet S, Lambert D, Portier X, Popov A, Kondratieva E, Goupil JM, Fallah El J, Gilson JP, Mariey L, Travert A, Mague F (2011) Effect of water on the stability of Mo and CoMo hydrodeoxygenation catalysts: a combined experimental and DFT study. J Catal 282:155–164
Deepa AK, Dhepe PL (2014) Function of metals and supports on the hydrodeoxygenaiton of phenolic compounds. ChemPlusChem 79:1573–1583
Lee WS, Wang Z, Wu RJ, Bhan A (2014) Selective vapor-phase hydrodeoxygenation of anisole to benzene on molybdenum carbide catalysts. J Catal 319:44–53
Prasomsri T, Shetty M, Murugappan K, Roman Leshkov Y (2014) Insights into the catalytic activity and surface modification of MoO3 during the hydrodeoxygenation of lignin-derived model compounds into aromatic hydrocarbons under low hydrogen pressures. Energy Environ Sci 7:2660–2669
Haario H (2007) MODEST users guide. Profmath Oy, Helsinki
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sulman, A., Mäki-Arvela, P., Bomont, L. et al. Vanillin Hydrodeoxygenation: Kinetic Modelling and Solvent Effect. Catal Lett 148, 2856–2868 (2018). https://doi.org/10.1007/s10562-018-2478-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-018-2478-1