Abstract
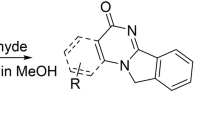
A common strategy to incorporate four-membered ring system between benzimidazole and quinoline cores was developed by one-pot protocol involving the condensation of o-phenylenediamine with 2-chloroquinoline-3-carbaldehyde derivatives followed by intramolecular palladium catalyzed C–N coupling. A series of ligands, palladium sources, bases and solvents were screened to optimize the reaction conditions for the synthesis of quinoline-fused azeto[1,2-a]benzimidazoles.
Graphical Abstract
An efficient catalytic system for the introduction of 4-membered-ring system between benzimidazole and quinoline cores has been developed by one-pot intramolecular C–N coupling of o-phenylenediamine with 6-substituted-2-chloroquinoline-3-carbaldehydes.



Similar content being viewed by others
References
Yadav S, Sinha D, Singh SK, Singh VK (2012) Chem Biol Drug Des 80:625
Husain A, Rashid M, Shaharyar M, Siddiqui AA, Mishra R (2013) Eur J Med Chem 62:785
El Rashedy AA, Aboul-Enein HY (2013) Mini Rev Med Chem 13:399
Göker H, Ertan R, Akgün H, Yulug N (1991) Arch Pharm 324:283
Bai Y-B, Zhang A-J, Tang J-J, Gao J-M (2013) J Agric Food Chem 61:2789
Parikh K, Joshi D (2013) Med Chem Res 22:3688
Kumar K, Awasthi D, Lee S-Y, Cummings JE, Knudson SE, Slayden RA, Ojima I (2013) Bioorg Med Chem 21:3318
Patel RV, Patel PK, Kumari P, Rajani DP, Chikhalia KH (2012) Eur J Med Chem 53:41
Gill C, Jadhav G, Shaikh M, Kale R, Ghawalkar A, Nagargoje D, Shiradkar M (2008) Bioorg Med Chem Lett 18:6244
Pieroni M, Tipparaju SK, Lun S, Song Y, Sturm AW, Bishai WR, Kozikowski AP (2011) ChemMedChem 6:334
Kumar K, Awasthi D, Lee S-Y, Zanardi I, Ruzsicska B, Knudson S, Tonge PJ, Slayden RA, Ojima I (2011) J Med Chem 54:374
Miller JF, Turner EM, Gudmundsson KS, Jenkinson S, Spaltenstein A, Thomson M, Wheelan P (2010) Bioorg Med Chem Lett 20:2125
Jain P, Sharma PK, Rajak H, Pawar RS, Patil UK, Singour PK (2010) Arch Pharm 33:971
Bhrigu B, Siddiqui N, Pathak D, Alam MS, Ali R, Azad B (2012) Acta Pol Pharm 69:53
Sondhi SM, Singh N, Kumar A, Lozach O, Meijer L (2006) Bioorg Med Chem 14:3758
Dixit S, Sharma PK, Kaushik N (2013) Med Chem Res 22:900
Achar KCS, Hosamani KM, Seetharamareddy HR (2010) Eur J Med Chem 45:2048
Chen G, Liu Z, Zhang Y, Shan X, Jiang L, Zhao Y, He W, Feng Z, Yang S, Liang G (2013) Med Chem Lett 4:69
Vandekerckhove S, De Moor S, Segers D, de Kock C, Smith PJ, Chibale K, De Kimpe N, D’hooghe M (2013) Med Chem Commun 4:724
Bhat HR, Singh UP, Gahtori P, Ghosh SK, Gogoi K, Prakashe A, Singh RK (2013) New J Chem 37:2654
Teguh SC, Klonis N, Duffy S, Lucantoni L, Avery VM, Hutton CA, Baell JB, Tilley L (2013) J Med Chem 56:6200
Solomon VR, Lee H (2011) Curr Med Chem 18:1488
Yamato M, Takeuchi Y, Hashigaki K, Ikeda Y, Chang MR, Takeuchi K, Matsushima M, Tsuruo T, Tashiro T (1989) J Med Chem 32:1295
Ghorab MM, Al-Said MS, El-Hossary EM (2011) J Heterocycl Chem 48:563
Chen Y-W, Chen Y-L, Tseng C-H, Liang C-C, Yang C-N, Yao Y-C, Lu P-J, Tzeng C-C (2011) J Med Chem 54:4446
Musiol R, Jampilek J, Buchta V, Silva L, Niedbala H, Podeszwa B, Palka A, Majerz-Maniecka K, Oleksynd B, Polanski J (2006) Bioorg Med Chem 14:3592
Bhat HR, Gupta SK, Singh UP (2012) RSC Adv 2:12690
Selvi ST, Nadaraj V, Mohan S, Sasi R, Hema M (2006) Bioorg Med Chem 14:3896
Upadhayaya RS, Vandavasi JK, Vasireddy NR, Sharma V, Dixit SS, Chattopadhyaya J (2009) Bioorg Med Chem 17:2830
Praveen C, DheenKumar P, Muralidharan D, Perumal PT (2010) Bioorg Med Chem Lett 20:7292
Das P, Deng X, Zhang L, Roth MG, Fontoura BMA, Phillips MA, De Brabander JK (2013) Med Chem Lett 4:517
Brandi A, Cicchi S, Cordero FM (2008) Chem Rev 108:3988
Wolfe JP, Wagaw S, Marcoux JF, Buchwald SL (1998) Acc Chem Res 31:805
Patel AB, Chikhalia KH, Kumari P (2014) Eur J Med Chem. doi:10.1016/j.ejmech.2014.03.085
Alsabeh PG, Lundgren RJ, Longobardi LE, Stradiotto M (2011) Chem Commun 47:6936
Patel AB, Chikhalia KH, Kumari P (2014) Res Chem Intermed. doi:10.1007/s11164-013-1377-8
Boger DL, Duff SR, Panek JS, Yasuda MJJ (1986) Org Chem 50:5782
Lindley J (1984) Tetrahedron 40:1433
Old DW, Harris MC, Buchwald SL (2000) Org Lett 2:1403
Fors BP, Krattiger P, Strieter E, Buchwald SL (2008) Org Lett 10:3505
Rocca P, Marsais F, Godard A, Queguiner G (1993) Tetrahedron 49:49
Alberico D, Scott ME, Lautens M (2007) Chem Rev 107:174
Fors BP, Buchwald SL (2010) J Am Chem Soc 132:15914
Cho SH, Yoon J, Chang S (2011) J Am Chem Soc 133:5996
Zhu L, Ye Y-M, Shao L-X (2014) Tetrahedron 68:2414
Yin J, Buchwald SL (2000) Org Lett 2:1101
Phukan K, Ganguly M, Devi N (2009) Synth Commun 39:2694
Shrivastava A, Singh RM (2005) Indian J Chem 44B:1868
Yin J, Buchwald SL (2002) J Am Chem Soc 124:6043
Cai D, Payack JF, Bender DR, Hughes DL, Verhoeven TR, Reider PJ (1994) J Org Chem 59:7180
McLaughlin MG, Cook MJ (2011) Chem Commun 47:11104
Borah M, Bhattacharyya PK, Das P (2012) Appl Organomet Chem 26:130
Chen G-F, Shen H-D, Jia H-M, Zhang L-Y, Kang H-Y, Qi Q-Q, Chen BH, Cao J-L, Li J-T (2013) Aust J Chem 66:262
Leutbecher H, Constantin M-A, Mika S, Conrad J, Beifuss U (2011) Tetrahedron Lett 52:605
Lin S, Yang L (2005) Tetrahedron Lett 46:4315
Guram AS, Buchwald SL (1994) J Am Chem Soc 116:7901
Paul F, Patt J, Hartwig JF (1994) J Am Chem Soc 116:5969
Acknowledgments
The authors are thankful to Director, S. V. National Institute of Technology, Surat for the scholarship, encouragement and facilities. We are also grateful to SAIF Punjab University, SDPARC, Kim and Centre of Excellence, Vapi, Gujarat, India, for providing necessary analytical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, A.B., Kumari, P. & Chikhalia, K.H. One-Pot Synthesis of Novel Quinoline-Fused Azeto[1,2-a]benzimidazole Analogs Via Intramolecular Pd-Catalyzed C–N Coupling. Catal Lett 144, 1332–1338 (2014). https://doi.org/10.1007/s10562-014-1266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1266-9