Abstract
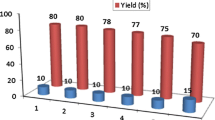
Paal–Knorr pyrrole synthesis was performed in the presence of superparamagnetic nanoparticles of modified sulfuric acid (γ-Fe2O3@SiO2-OSO3H) as an efficient and magnetically separable catalyst. Recovery of the catalyst was simple using a magnet, allowing its reuse without significant loss of its catalytic activity (over five cycles).
Graphical Abstract



Similar content being viewed by others
References
Jones RA, Bean GP (1977) Pyrrolchemie: the chemistry of pyrroles. Academic Press, London
Furstner A (2003) Angew Chem Int Ed 42:3528
De Leo CY, Ganem B (1997) Tetrahedron 53:7731
Satyanarayana VSV, Sivakumar A (2011) Ultrason Sonochem 18:922
Abbas T, Najafi CA (2012) Chin J Chem 30:372
Li D, Zang H, Wu C, Yu N (2013) Ultrason Sonochem 20:1144
Raghavan S, Anuradha K (2003) Synlett 2003:711
Darabi HR, Poorheravi MR, Aghapoor K, Mirzaee A, Mohsenzadeh F, Asadollahnejad N, Taherzadeh H, Balavar Y (2012) Environ Chem Lett 12:5
Mohammed A, Andrew S, Bela T (2006) Adv Synth Catal 348:2191
Banik BK, Samajdar S, Banik I (2004) J Org Chem 69:213
Chen J, Wu H, Zheng Z, Jin C, Zhang X, Su W (2006) Tetrahedron Lett 47:538
Veisi H (2010) Tetrahedron Lett 51:2109
Banik BK, Banik I, Renteria M, Dasgupta SK (2005) Tetrahedron Lett 46:2643
Darabi HR, Aghpoor K, Darestani Farahani A, Mohsenzadeh F (2012) Environ Chem Lett 10:369–375
Surya KD (2008) Catal Lett 124:174
Rahmatpour A (2012) J Organomet Chem 712:15
Armugam P, Perumal PT (2006) Chem Lett 35:632
Rahmatpour A (2012) Monatsh Chem 143:491
Curini M, Montanari F, Rosati O, Lioy E, Margarita R (2003) Tetrahedron Lett 44:3923
Aghapoor K, Ebadi-Nia L, Mohsenzadeh F, Mohebi F (2012) Morad, Y. Balavar, H. R. Darabi. J Organomet Chem 708–709:25
Zhan ZH, Li JJ, Li TS (2008) Ultrason Sonochem 15:673
Danks TN (1999) Tetrahedron Lett 40:3957
Handy ST, Lavender K (2013) Tetrahedron Lett 54:4377
Stevens PD, Li G, Fan F, Yen M, Gao Y (2005) Chem Commun 41:4435
Rostamnia S, Lamei K, Mohammadquli M, Sheykhan M, Heydari A (2012) Tetrahedron Lett 53:5257
Sheykhan M, Ma’mani L, Ebrahimi A, Heydari A (2011) J Mol Catal A 335:253
Ma’mani L, Sheykhan M, Heydari A (2011) Appl Catal A 395:34–38
Ma’mani L, Heydari A, Sheykhan M (2010) Appl Catal A 384:122–127
Sheykhan M, Mohammadquli M, Heydari A (2012) J Mol Struct 1027:156–161
Acknowledgments
The authors would like to acknowledge financial support provided by Tarbiat Modares University for carrying out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheraghi, S., Saberi, D. & Heydari, A. Nanomagnetically Modified Sulfuric Acid (γ-Fe2O3@SiO2-OSO3H): An Efficient, Fast, and Reusable Catalyst for Greener Paal–Knorr Pyrrole Synthesis. Catal Lett 144, 1339–1343 (2014). https://doi.org/10.1007/s10562-014-1197-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1197-5