Abstract
The use of pyridinium ionic liquids as solvent and catalytic systems based on yttrium salts dissolved in pyridinium ionic liquids for the synthetically important Diels–Alder reaction between cyclopentadiene and ethyl-vinyl ketone was studied. Both chloride and triflate were active catalysts in cycloaddition, strongly improving the reaction rate and stereoselectivity. The catalytic system—1 mol% YCl3/[C4-3-C1py][OTf] (N-butyl-3-methylpyridinium trifluoromethanesulfonate) proved to be stable under recycling conditions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ionic liquids seem to be promising reaction media for organic reactions [1, 2], because of their unique properties desirable for ideal solvents, such as low volatility, nonflamability, good thermal stability and recyclability [3].
The first pyridinium ionic liquids used as solvents in Diels–Alder reaction were 1-ethylpyridinium tetrafluoroborate and 1-ethylpyridinium trifloroacetate, investigated in the cycloadditions of isoprene with dienophiles: acrylonitrile, acrylic acid and methacrylic acid [4]. The ionic liquid 1-ethylpyridinium trifloroacetate was found to be an excellent reaction solvent enhancing both, selectivity and reaction rate. N-hexylpyridinium bis(trifluoromethylsulfonyl)imide, had been tested before as a reaction medium in the intermolecular Diels–Alder reaction [5].
Moreover Diels–Alder reactions in the catalytic systems based on 1, 5, 10 and 20 mol% of Er(OTf)3 immobilized in 1-methylpyridinium trifluoromethanesulfonate were studied [6]. The addition of a catalyst enhanced significantly the reaction rates of the cycloadditions of cyclopentadiene and two dienophiles: acrolein and ethyl acrylate.
Catalytic systems based on metal triflates or chlorides and N-hexylpyridinium bistriflimide were found to be active in Diels–Alder reaction. They were suitable to obtain 2-ethoxycarbonyl-5-norbornene, 2,3-bis(methoxycarbonyl)-5-norbornene and 2-etanoyl-5-norbornene [7]. Catalytic system Mg(OTf)2 and N-hexylpyridinium bistriflimide was found to be stable under recycling conditions in synthesis of 2,3-bis(methoxycarbonyl)-5-norbornene [8].
Encouraged by these promising results we used new catalytic systems, based on pyridinium ionic liquids and yttrium salts—Y(OTf)3 and YCl3. Generally trifluoromethanesulfonates (triflates) are well known for catalytic activity in many organic reactions [9] also in Diels–Alder reaction. After their discovery, the first ones such as yttrium, scandium and ytterbium triflates were used by Kobayashi in cycloaddition of isoprene and various dienophiles [10]. After that application, many triflates, such as cerium, bismuth, indium, lanthanium, erbium were used in cycloaddition [11–16] also in ionic liquids as solvents.
2 Experimental
2.1 Materials
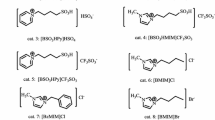
Ionic liquids, such as [C2py][NTf2] (N-ethylpyridinium bis(trifluoromethylsulfonyl)imide, CAS 712354-97-7), [C4py][NTf2] (N-butylpyridinium bis(trifluoromethylsulfonyl)imide, CAS 187863-42-9), [C4-3-C1py][NTf2] (N-butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)-imide, CAS 344790-86-9), [C4-3-C1py][OTf] (N-butyl-3-methylpyridinium trifluoromethanesulfonate, CAS 857841-32-8), [C4-3-C1py][BF4] (N-butyl-3-methylpyridinium tetrafluoroborate, CAS 597581-48-1) were purchased from Iolitec. [C6py][NTf2] (N-hexylpyridinium bis(trifluoromethylsulfonyl)imide, CAS 460983-97-5) was purchased from Aldrich and [C4-3-C1py][FAP] N-butyl-3-methylpyridinium tris(pentafluoroethyl)trifluorophosphate from Merck. Ionic liquids were of high purity grade with quoted purity of mass fraction 0.99 determined by NMR. Prior to their use, the ionic liquids were washed with the deionized water several times (until the test for chloride content performed with AgNO3 titration was negative). Furthermore, they were dried in a vacuum drier at 323 K and 5 mbar for 24 h. Next the water content was measured by Karl Fisher titration.
Dicyclopentadiene of 95% purity was bought at Fluka. Ethyl-vinyl ketone ≥95%, anhydrous yttrium chloride (99.99%) and yttrium trifluoromethanesulfonate (99.99%) was purchased from Aldrich.
2.2 Procedure for Diels–Alder Reaction
The reactions were carried out in 4 mL vials. A mixture of a given ionic liquid 0.25 mL, ethyl-vinyl ketone (1 mmol), cyclohexanone (20 μL) as an internal chromatographic standard and freshly cracked cold cyclopentadiene (1.5 mmol) was added. Freshly cracked cold cyclopentadiene (1.5 mmol) was added to the mixture of a given ionic liquid (0.25 mL), ethyl-vinyl ketone (1 mmol), cyclohexanone (20 μL) used as an internal chromatographic standard. The reaction was conducted at 25 °C. The progress of the reaction was monitored by GC analysis over the time needed to obtain quantitative yield. As a result a mixture of endo and exo isomers: 2-propanoyl-5-norbornene was obtained. The yield of products and endo:exo ratios were calculated on the basis of GC analysis.
If the reaction was to be performed in the presence of a catalyst (Y(OTf)3 or YCl3) the required portion of the catalyst was weighted first (0.01 mmol) and dissolved in the ionic liquid.
2.3 Analysis
The GC analysis was carried out using a Carlo Erba GC 8000 TOP apparatus equipped with a FID detector, RXI®-17 column: 30 m long with diameter of 0.53 mm and 1.5 μm film layer column (Restek). The column temperature was programmed as follows: at the moment of injection the temperature was increased at the rate 10 °C/min, it was kept constant at 120 °C for 2 min, then it was increased at the rate 20 °C/min and was kept constant at 240 °C for 20 min, and finally it was decreased to 80 °C. The detector temperature was 260 °C. The quantitative analysis was performed according to the internal standard method.
2.4 Method for Calculating Reaction Rate Constant
The method of calculating reaction rate constant was shown by the example of Diels–Alder reaction between cyclopentadiene and ethyl-vinyl ketone carried out in ionic liquid ([C4py][NTf2]). Studied cycloadditions are second-order reactions, first order with respect to each reactant. In terms of the variable x representing the decrease in concentration of a reactant in a given time reaction rate is given by:
where: \( C_{\text{A}}^{0} \)—initial concentration of cyclopentadiene, \( C_{\text{B}}^{0} \)—initial concentration of ethyl-vinyl ketone.
The concentration of the reagents the beginning of the reaction is not equal \( C_{\text{A}}^{0} \ne C_{\text{B}}^{0} \), so integrated form of the equation is
where \( C_{\text{A}}^{{}} ,\,\,C_{\text{B}}^{{}} \)—concentration of cyclopentadiene and ethyl-vinyl ketone at time t, respectively. Reaction rate constants were determined by dividing the slope of the plot \( { \ln }\frac{{C_{\text{B}}^{0} \cdot C_{\text{A}}^{{}} }}{{C_{\text{A}}^{0} \cdot C_{\text{B}}^{{}} }} \) against time t by (\( C_{\text{A}}^{0} - C_{\text{B}}^{0} \)). As an example, changes in the function of \( { \ln }\frac{{C_{\text{B}}^{0} \cdot C_{\text{A}}^{{}} }}{{C_{\text{A}}^{0} \cdot C_{\text{B}}^{{}} }} \) against t in cycloaddition between cyclopentadiene and ethyl-vinyl ketone carried out in [C4py][NTf2] were shown in Fig. 1.
Calculated reaction rate constants in cycloadditions carried out in all pyridinium ionic liquids and correlation coefficients were show in Table 1.
3 Results and Discussion
Diels–Alder reaction between cyclopentadiene and ethyl-vinyl ketone was investigated in ionic liquids as reaction media at 25 °C. Calculated reaction rate constants based on the method described in sect. 2.4 were presented in Table 1. Ketones are generally the most active dienophiles in Diels–Alder cycloaddition owing the positive effect that the carbonyl group has on the reaction by lowering the energy of LUMO orbital of ketone. Reaction rate constants are much higher in the cycloadditions of ketones with cyclopentadiene than in reactions of other dienophiles. For example in Diels–Alder reaction between cyclopentadiene and esters, such as methyl acrylate, ethyl acrylate or butyl acrylate [17] performed in ionic liquids such as imidazolium tetrafluoroboranes or hexafluorophosphates the reaction rate constants were from 4 to 5 times lower than those presented in Table 1.
In all studied ionic liquids Diels–Alder reaction was completed within 2 h. Although the reaction rate constant was the highest in [C4-3-C1py][FAP], generally ionic liquids with [NTf2] anion were found to accelerate the reaction much more than ionic liquids with [OTf] or [BF4] anion. Also the reaction rate constant increased with the complexity of the anion, probably due to higher negative charge delocalization around the anion, which weakens the cation–anion interactions within ionic liquid. In this way the cation might be more involved into promoting cycloaddition. Moreover less polar, according to empirical polarity scale—ET parameter value pyridinium ionic liquids were accelerating the Diels–Alder reaction much more. However [C4-3-C1py][FAP] was found to be an exception. Stereoselectivity was found to be independent on the type of ionic liquid. The endo:exo ratios were comparable in all used reaction media.
After 5 min in a catalytic system made of 1 mol% of Y(OTf)3 and ionic liquid [C2py][NTf2] or [C4py][NTf2], the conversion of ethyl-vinyl ketone was 98% (Fig. 2a). In the catalytic system based on yttrium triflate and [C6py][NTf2]—ionic liquid, showing the highest dynamic viscosity, lowest density and surface tension, the ethyl-vinyl ketone conversion was slower. As long as 30 min was necessary to obtain 96% of ethyl-vinyl ketone conversion. However, it was observed that dienophile’s conversion was higher in N-butylpyridinium than in N-butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imides. This result means that the methyl substituent in pyridinium ring causes a decrease in the rate of cycloaddition.
In the group of N-butyl-3-methylpyridinium ionic liquids, the type of anion influenced the reaction rate. The fastest cycloaddition was observed in the ionic liquid with the highest anion’s complexity which is [C4-3-C1py][NTf2] (Fig. 2b). Moreover, this ionic liquid has the lowest dynamic viscosity, surface tension and surface excess entropy from among all ionic liquids with the same cation, which can explain the observed increase in the reaction rate. Stereoselectivity in the catalytic systems with Y(OTf)3 was the highest in N-butyl-3-methylpyridinium ionic liquids: 17.4 in [C4-3-C1py][BF4], 10.6 in [C4-3-C1py][FAP] and 10.4 in [C4-3-C1py][NTf2] (Fig. 4).
In the catalytic systems—1 mol% YCl3/ionic liquid the reaction ran faster in the group of ionic liquids with N-butyl-3-methylpyridinium cation (Fig. 3b). The most active was the system containing the ionic liquid with bis(trifluoromethylsulfonyl)imide (NTf2) anion. After 5 min the conversion of ethyl-vinyl ketone was 95%. Very fast was also the reaction carried in 1 mol% YCl3/[C4-3-C1py][OTf]. To obtain 92% of dienophile’s conversion 20 min was required. The same time was necessary to achieve 90% of ethyl-vinyl ketone conversion in [C6py][NTf2]. In the group of N-alkylpyridinium bis(trifluoromethylsulfonyl) imides the cycloaddition ran faster in ionic liquids that had longer alkyl chain in pyridinium cation (Fig. 3a). So YCl3 as a catalyst was more active in ionic liquids with higher dynamic viscosity and lower density and surface tension, which is completely the opposite to the observation made for the catalytic systems based on Y(OTf)3 and N-alkylpyridinium bis(trifluoromethylsulfonyl)imides. The most stereoselective was the system YCl3/[C4-3-C1py][NTf2] (Fig. 4). The stereoselectivity was found to decrease in the systems taking into account ionic liquids’ anion in the order [NTf2] < [OTf] < [BF4] < [FAP].
Although in Diels–Alder reaction between ethyl-vinyl ketone and cyclopentadiene the most active were the catalytic systems based on yttrium(III) trifluoromethanesulfonate, in recycling studies the systems based on chlorides were more suitable. Y(OTf)3 was too active in accelerating oligomerization of cyclopentadiene which was competitive to the reaction leading to 2-propanoyl-5-norbornene. High activity of Y(OTf)3 was described before [7, 18]. Moreover yttrium(III) chloride as a catalyst was more stereoselective in the studied cycloaddition. For recycling studies, two catalytic systems were chosen consisting of 0.02 mmol of YCl3 and 2 mmol of an ionic liquid either [C4-3-C1py][NTf2] or [C4-3-C1py][OTf]. The substrates were as follows: 2 mmol of ethyl-vinyl ketone and 3 mmol of cyclopentadiene, so the catalyst content was 1 mol% relative to that of dienophile, like in the reactions studied previously. Recycling was made by distillation of the product and unconverted substrates after the reaction under reduced pressure. There was no threat that the ionic liquids might decompose at high temperature, because previous thermogravimetric studies proved them to be thermally stable up to 500 K.
The catalytic system—1 mol% YCl3/[C4-3-C1py][NTf2] provided a high yield in the first four cycles (Fig. 5). However it became subsequently less active in further cycloaddition. In the 6th cycle the yield of the product after 5 min was only 61%. After each cycle a decrease in stereoselectivity was observed. At first the endo:exo ratio of isomers was 12.4 and in the 7th cycle it decreased to 6.5. Moreover, the catalytic system was becoming darker after each cycle because of the polymers formed in a reaction competitive to that of Diels–Alder. Even extraction with n-hexane of the system after the 6th run did not improve the catalytic activity of the examined system.
More stable in the recycling condition was the second studied catalytic system (Fig. 6). It was observed that in 1 mol% YCl3/[C4-3-C1py][OTf], the yield was higher than 93% after eight cycles. Moreover, stereoselectivity remained high for a long time, it decreased from 12.4 in the 1st run to 11.4 in the 9th, which was twice as high as the value obtained in this particular ionic liquid without a catalyst. Long lasting catalytic activity of 1 mol% YCl3/[C4-3-C1py][OTf] in recycling conditions proved its stability.
4 Conclusions
Catalytic systems based on yttrium salts and pyridinium ionic liquids were found to be active in obtaining 2-propanoyl-5-norbornene. However yttrium trifluoromethanesulfonate was accelerating the undesirable reaction as well, so yttrium chloride as catalyst is more recommended. More stable in recycling conditions was the catalytic system YCl3/[C4-3-C1py][OTf]. It maintained high activity in Diels–Alder reaction in nine subsequent cycles. Because of the possibility of working in the ambient reaction condition, easy product’s separation, high activity, meant as high conversion of substrate and high stereoselectivity, the mentioned catalytic system was found very useful in synthesis of norbornene derivatives.
References
Yang Z, Pan W (2005) Enzyme Microb Technol 37:19–28
Earle MJ, Seddon KR (2000) Pure Appl Chem 72:1391–1398
Wilkes JS (2004) J Mol Catal A Chem 214:11–17
Xiao Y, Malhotra SV (2004) Tetrahedron Lett 45:8339–8342
Tiwari S, Khupse N, Kumar A (2008) J Org Chem 73:9075–9083
Bortolini OD, Nino A, Garofalo A, Maiuolo L, Procopio A, Russo B (2010) Appl Catal A 372:124–129
Bittner B, Janus E, Milchert E (2010) Cent Eur J Chem 9:192–198
Bittner B, Milchert E, Janus E (2010) Pol J Chem Technol 12:3–5
Luo S, Zhu L, Talukdar A, Zhang G, Mi X, Cheng J, Wang PG (2005) Mini Rev Org Chem 2:546–564
Kobayashi S, Hachiya I, Araki M, Ishitani H (1993) Tetrahedron Lett 34:1755–1758
Fukuzawa S, Metoki K, Esumi S (2003) Tetrahedron 59:10445–10452
Pinto RMA, Salvador JAR, Le Roux C (2008) Catal Commun 9:465–469
Song CE, Shim WH, Roh EJ, Lee SG, Choi JH (2001) Chem Commun 1122–1123
Sarma D, Kumar A (2008) Appl Catal A Gen 335:1–6
Vidis A, Kusters E, Sedelmeier G, Dyson PJ (2008) J Phys Org Chem 21:264–270
Silvero G, Arevalo MJ, Bravo JL, Avalos M, Jimenez JL, Lopez I (2005) Tetrahedron 61:7105–7111
Tiwari S, Kumar A (2006) Angew Chem 118:4942–4943
Janus E, Bittner B (2010) Catal Lett 134:147–154
Acknowledgments
This scientific work was financed by Ministry of Science and Higher Education as project No. N N 209 106239.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Bittner, B., Pełech, R., Janus, E. et al. Synthesis of 2-Propanoyl-5-Norbornene in Pyridinium Ionic Liquids Catalyzed by Yttrium Salts. Catal Lett 142, 332–337 (2012). https://doi.org/10.1007/s10562-012-0772-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-012-0772-x