Abstract
In this letter, a series of bulky N-acyl-pyridylbenzamidine ligands were synthesized and characterized. Under the optimized reaction conditions, these bulky ligands were applied to catalyze Suzuki–Miyaura reactions of various aryl bromides and chlorides. The desired biaryl products were obtained in good to high yields.
Graphical Abstract
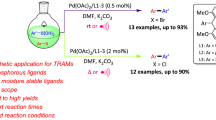
A series of bulky N-acyl-pyridylbenzamidine ligands were synthesized and employed in palladium catalyzed Suzuki–Miyaura reactions. This catalytic system showed effective catalytic efficiency towards an array of various aryl bromides and chlorides under aerobic conditions.



Similar content being viewed by others
References
Suzuki A (1982) Acc Chem Res 15:178
Suzuki A (1994) Pure Appl Chem 66:213
Miyaura N, Suzuki A (1995) Chem Rev 95:2457
Stanforth SP (1998) Tetrahedron 54:263
Chen Y, Wu CS (2009) Macromolecules 42:3729
Spencer J, Baltus CB, Patel H, Press NJ, Callear SK, Male L, Coles SJ (2011) Comb Sci 13:24
Dupont J, Consorti CS, Spencer J (2005) Chem Rev 105:2527
Liebscher J, Yin LX (2007) Chem Rev 107:133
Guiry PJ, Hargaden GC (2009) Chem Rev 109:2505
So CM, Lau CP, Kwong FY (2007) Org Lett 9:2795
So CM, Chan ASC, Kwong FK (2008) J Org Chem 73:7731
Yang DX, Colletti SL, Wu K, Song MY, Shen HC (2009) Org Lett 11:381
Baillie C, Zhang LX, Xiao JL (2004) J Org Chem 69:7779
Iwasana T, Komano T, Tajima A, Tokunaga M, Obata Y, Fujihara T, Tsuji Y (2006) Organometallics 25:4665
Plentio H, Fleckenstein CA (2008) Organometallics 27:3924
Kantam ML, Srinivas P, Yadar J, Likhar PR, Bhargava S (2009) J Org Chem 74:4882
Li JH, Liu WJ (2004) Org Lett 6:2809
Mino T, Shirae Y, Sakamoto M, Fujita T (2005) J Org Chem 70:2191
Li SH, Lin YJ, Gao JG, Zhang SB (2007) J Org Chem 72:4067
Marion N, Nolan SP (2008) Acc Chem Res 41:1440
Chalker JM, Wood CS, Davis BG (2009) J Am Chem Soc 131:16346
Zhou ZZ, Liu FS, Shen DS, Tan C, Luo LY (2011) Inorg Chem Commun 14:659
Sinner F, Buchmeiser MR, Tessadri R, Mupa M, Wurst K, Bonn GK (1998) J Am Chem Soc 120:2790
Buchmeiser MR, Wurst K (1999) J Am Chem Soc 121:11101
Buchmeiser MR, Schareina T, Kempe R, Wurst K (2001) J Organomet Chem 634:39
Silberg J, Schareina T, Wurst K, Buchmeiser MR (2001) J Organomet Chem 622:6
Buchmeiser MR (2002) Bioorg Med Chem Lett 12:1837
Nájera C, Gil-Moltó J, Karlström S (2004) Adv Synth Catal 346:1798
Nájera C, Gil-Moltó J, Karlström S, Falvello LR (2003) Org Lett 5:1451
Gil-Moltó J, Karlström S, Nájera C (2005) Tetrahedron 61:12168
Gil-Moltó J, Nájera C (2005) Eur J Org Chem 4073
Liu FS, Gao HY, Song KM, Zhang L, Zhu FM, Wu Q (2009) Polyhedron 28:1386
Zhang HC, Kwong FY, Tian Y, Chan KS (1998) J Org Chem 63:6886
Braga AAC, Morgon NH, Ujaque G, Maseras F (2005) J Am Chem Soc 127:9298
Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M (2002) Chem Rev 102:1359
Adamo C, Amatore C, Ciofini I, Jutand A, Lakmini H (2006) J Am Chem Soc 128:6829
Milstein D, Portnoy M (1993) Organometallics 12:1665
Ahlquist M, Norrby PO (2007) Organometallics 26:550
Ochi N, Nakao Y, Sato H, Mantano Y, Imahori H, Sakaki S (2009) J Am Chem Soc 131:10955
Acknowledgements
We are sincerely grateful for the financial support from the National Natural Science Foundation of China (No. 21004014).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, C., Liu, FS., Shen, DS. et al. Bulky N-acyl-pyridylbenzamidine Ligands for Palladium Catalyzed Suzuki–Miyaura Reaction. Catal Lett 141, 1332–1337 (2011). https://doi.org/10.1007/s10562-011-0625-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0625-z