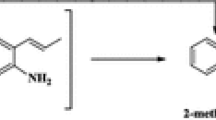Abstract
The conversion of anisole, a prototypical bio-oil compound, was catalyzed by Pt/Al2O3 in the presence of H2 at 573 K, with the selectivity for C–O bond breaking approximately matching that for ring hydrogenation; these reactions were accompanied by methyl group transfers matching those in the conversion catalyzed by HY zeolite.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant biomass is a potentially valuable renewable source of fuels and chemicals. Lignocellulose can be rapidly transformed into liquids by pyrolysis, giving “bio-oil,” but the high oxygen content leads to immiscibility with fossil fuels, instability, and corrosion [1–3]. Bio-oils typically contain several hundred compounds [4, 5], and an understanding of the reaction networks in their catalytic upgrading is limited; even information characterizing the upgrading of individual bio-oil compounds is scarce [6–10]. Our approach to begin unravelling this chemistry, and specifically to understand oxygen removal reactions, was to investigate the conversion of a bio-oil compound, anisole, chosen as a prototype because it has both an ether linkage and an aromatic ring. Reactions were catalyzed by a solid acid (HY zeolite, Zeolyst CBV 720) and separately by a catalyst consisting of a metal dispersed on an acidic support (Pt/Al2O3, Sigma–Aldrich, 1% wt Pt). The goal of the research was to develop detailed reaction networks, characterized by numerous connected pathways, for the conversion of anisole with catalysts representing these two important classes of catalysts.
2 Experimental
Catalytic reactions were carried out in a once-through packed-bed flow reactor under the following conditions, with liquid anisole (methoxybenzene, Sigma–Aldrich, 99.8%) vaporized into a flowing gas stream: catalyst mass 4.1–401.5 mg, temperature 573 K, pressure 140 kPa, anisole liquid flow rate 0.03 mL min−1, gas feed flow rate 100 mL min−1 (as pure N2 in experiments with HY zeolite and as 30% H2/70% N2 in experiments with Pt/Al2O3). Each catalyst powder was diluted with particles of inert, nonporous α-Al2O3. The product gas stream was condensed at 285–288 K. Uncondensed gases and condensate samples collected periodically were analyzed by gas chromatography and gas chromatography/mass spectrometry.
3 Results
Dozens of products were observed in the conversions with each catalyst. Significant catalyst deactivation, characterized by a 50% reduction in conversion within 1 h on stream, was observed with HY zeolite; the Pt/Al2O3 catalyst working in the presence of H2 was much more stable. Selectivities to major and minor products at low initial conversions (0.14 for Pt/Al2O3 and 0.16 for HY zeolite) are summarized in Table 1; these data represent extrapolations to zero time on stream and therefore initial catalyst performance, prior to significant deactivation.
The most abundant products of the conversion catalyzed by HY zeolite were phenol, methylanisoles, and methylphenols; less abundant products included dimethylanisoles and dimethylphenols. The substitution at the 2- and 4-positions on the aromatic ring is consistent with the kinetically determined substitution preference of aromatic ethers and aromatic alcohols [11]. Trace products included those with additional methyl-group substitutions on the aromatic ring (observed with each catalyst) and hydrogenation products, such as cyclohexane, which were also formed with Pt/Al2O3 in the presence of H2.
The most abundant products of the reactions catalyzed by Pt/Al2O3 were phenol, 2-methylphenol, and benzene; less abundant products included water, methane, methanol, 2-methylanisole, and 4-methylanisole. The appearance of cyclohexanone, for example, indicates hydrogenation of the aromatic ring [12].
Thus, an important class of reaction, observed for both catalysts, is methyl group transfer. Hydrogenolysis, hydrogenation, and hydrodeoxygenation were also observed in the conversion catalyzed by Pt/Al2O3 in the presence of H2.
Selectivity versus conversion plots (e.g., Fig. 1) were used to identify products as primary or not [13, 14] (these designations are empirical, falling short, for example, of providing information about intermediates that were too reactive to be detected). The selectivity–conversion data observed for anisole conversion catalyzed by HY zeolite (Figs. 1, 2) indicate that phenol, 2-methylanisole, 4-methylanisole, and 2,6-dimethylphenol (data not shown) were primary products and that 2-methylphenol and 4-methylphenol (data not shown) were non-primary products. Benzene and cyclohexanone were not observed.
Selectivity for the formation of phenol (closed circles) and 2-methylphenol (open circles) in the conversion of anisole catalyzed by HY zeolite at 573 K. Data for each product were fitted with a straight line and extrapolated to zero conversion; intercepts of regression lines significantly different from zero selectivity at zero conversion (analyzed with 95% confidence limits) indicate primary products, in this case phenol, and those not significantly different from zero (analyzed with 95% confidence limits) are considered non-primary, in this case 2-methylphenol
Selectivity for the formation of 2-methylanisole (closed circles) and 4-methylanisole (open circles) in the conversion of anisole catalyzed by HY zeolite; conditions are stated in the text. Data for each product were fitted with a straight line and extrapolated to zero conversion; intercepts significantly different from zero selectivity at zero conversion (analyzed with 95% confidence limits) indicate primary products, in this case both 2-methylanisole and 4-methylanisole, and those not significantly different from zero are considered to be evidence of non-primary products
On the basis of the data showing that methyl group transfer (transalkylation) is the predominant class of reaction and a primary reaction in the formation of phenol, 2-methylanisole, and 4-methylanisole in the conversion catalyzed by HY zeolite, we infer the qualitative reaction network shown in Fig. 3. For simplicity, the source of the methyl group is not always shown in the reaction network.
Reaction network for the conversion of anisole catalyzed by HY zeolite at 573 K. Reactions are postulated on the basis of the products identified, including trace compounds, and their formation as a result of possible methyl group transfer reactions. Bold arrows show the reactions that are kinetically most significant (with the width of the arrow denoting a rough measure of rate; the wider the arrow, the faster the reaction)
The selectivity–conversion data observed for anisole conversion catalyzed by Pt/Al2O3 (Figs. 4, 5) in the presence of H2 indicate that phenol, 2-methylphenol, and 4-methylphenol (data not shown) were primary products and that benzene, cyclohexanone, 2- and 4-methylanisole, and 2,6-dimethylphenol (data not shown) were non-primary products.
Selectivity for the formation of several products in the conversion of anisole catalyzed by Pt/Al2O3 in the presence of H2 at 573 K. Data for each product were fitted with a straight line and analyzed with 95% confidence limits as indicated in the caption of Fig. 1; primary products in this case are phenol (open circles) and 2-methylphenol (closed triangles); benzene (closed circles) is a non-primary product
Selectivity for the formation of 2-methylanisole (closed circles) and cyclohexanone (open circles) in the conversion of anisole catalyzed by Pt/Al2O3; conditions are stated in the text. Data for each product were fitted with a straight line and extrapolated to zero conversion; intercepts significantly different from zero selectivity at zero conversion (analyzed with 95% confidence limits) indicate primary products, and those not significantly different from zero (analyzed with 95% confidence limits) are considered to be evidence of non-primary products, in this case 2-methylanisole and cyclohexanone
Presuming that methyl group transfer, hydrodeoxygenation, and hydrogenation are the important reaction classes, and by recognizing which compounds were primary products, we inferred the reaction network of Fig. 6 for anisole conversion catalyzed by Pt/Al2O3 in the presence of H2.
Reaction network for the conversion of anisole and hydrogen catalyzed by Pt/Al2O3 at 573 K. Hydrogenation/hydrodeoxygenation reactions are represented by dotted lines and methyl group transfer reactions by solid lines. H2 as a reactant is omitted for simplicity (designation of arrows as in Fig. 3)
The data characterizing the conversion of anisole catalyzed by Pt/Al2O3 are represented satisfactorily by first-order kinetics (Fig. 7). The pseudo first-order rate constant for the overall disappearance of anisole was found to be 19 L (g of catalyst · h) under the conditions stated in the caption of Fig. 6. Selectivities to benzene relative to cyclohexanone indicate that the rate of hydrogenolysis of the C–O bond approximately matched that of hydrogenation of the aromatic ring at low conversions and at the low pressure of these experiments.
4 Discussion
The reaction network of Fig. 3 accounts for major, minor, and trace products; it contains more detail than the reported reaction network proposed for conversion of anisole catalyzed by the zeolite HZSM-5 [6] and is broadly consistent with it. Individual compounds produced with HZSM-5 and not identified in our work include 3-methylphenol and methane, possibly indicating the higher reaction temperature (673 K) or higher inverse space velocity in the reported work.
Similarly, the reaction network of Fig. 6 accounts for the major, minor, and trace products in the reactions catalyzed by Pt/Al2O3, and, again, the detail markedly exceeds than of any comparable reaction network including catalytic hydrodeoxygenation. A comparison of Figs. 3, 6 shows that primary products formed in the presence of one catalyst (e.g., 2-methylphenol) are not necessarily primary products formed in the presence of the other catalyst. The low selectivity to benzene relative to that of products resulting from methyl group transfers suggests that higher concentrations of H2 (and high pressures) will be required to achieve acceptable yields and selectivities for oxygen removal.
5 Conclusions
In the conversion of anisole catalyzed by Pt/Al2O3 and by HY zeolite, methyl group transfers were found to be kinetically significant reactions, and when the catalyst was Pt/Al2O3 used in the presence of H2, the conversion was accompanied by products of C–O bond-breaking and hydrogenation reactions. Data such as those reported here provide a starting point for predicting the conversion of bio-oils, specifically for removal of oxygen and upgrading of fuel properties.
References
Huber GW, Iborra S, Corma A (2006) Chem Rev 106:4044
Mohan D, Pittman CU, Steele PH (2006) Energy Fuels 20:848
Stöcker S (2008) Angew Chem Int Ed Engl 47:9200
Branca C, Giudicianni G, DiBlasi C (2003) Ind Eng Chem Res 42:3190
Milne T, Agblevor F, Davis M, Deutch S, Johnson D (1997) In: Bridgwater AV, Boocock DGB (eds) Developments in thermal biomass conversion. Blackie, London, pp 409–424
Zhu X, Mallinson RG, Resasco DE (2010) Appl Catal A 379:172
Hong D-Y, Miller SJ, Agrawal PK, Jones C (2010) Chem Commun 46:1038
Gayubo AG, Aguayo AT, Atutxa A, Aguado R, Bilbao J (2004) Ind Eng Chem Res 43:2610
Gayubo AG, Aguayo AT, Atutxa A, Aguado R, Olazar R, Bilbao J (2004) Ind Eng Chem Res 43:2619
Elliott DC, Hart TR (2008) Energy Fuels 23:631
Streitwieser A Jr, Heathcock CH (1985) Introduction to Organic Chemistry. Macmillan, New York
Weissermel K, Arpe H-J (1997) Industrial organic chemistry. Wiley-VCH, New York
Bhore NA, Klein MT, Bischoff KB (1990) Ind Eng Chem Res 29:313
Bhore NA, Klein MT, Bischoff KB (1990) Chem Eng Sci 45:2109
Acknowledgments
Financial support for this work was provided by Chevron Technology Ventures, a division of Chevron USA, Inc. An Agilent Technologies Foundation Research Project Gift provided a GC7890 Refinery Gas Analyser.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Runnebaum, R.C., Nimmanwudipong, T., Block, D.E. et al. Catalytic Conversion of Anisole: Evidence of Oxygen Removal in Reactions with Hydrogen. Catal Lett 141, 817–820 (2011). https://doi.org/10.1007/s10562-010-0510-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0510-1