Abstract
Liquid phase hydrogenation of acetophenone (ACT) is studied over ruthenium catalysts (1-4 wt% Ru) supported on gel-type methacrylate-styrene resin (FCN) functionalized with C=O, –NH, and –NH2 groups. Microscopic studies (SEM, STEM) show that the nature of Ru particles depends on the level of Ru doping. At low Ru content (1–2 wt%) ruthenium nano-clusters are formed, while at 4 wt% Ru, metal crystallites of few nanometers in size are observed. Catalytic reactions are performed at mild conditions (atmospheric pressure of hydrogen, 40 °C) in biphasic isooctane-water (IO/H2O) solvent system, and, for comparison, in a single component solvent (isooctane or ethanol). The use of biphasic IO/H2O solvent system results in a dramatic improvement of selectivity. The hydrogenation of C=O in acetophenone dominates over the hydrogenation of aromatic ring, yielding 1-phenylethanol with ca. 80% selectivity. Ru/FCN catalysts exhibit higher selectivity than the reference Ru/Al2O3. This is tentatively assigned to the steric effects induced by the polymer network on migrating reactant molecules. Advantageous influence of biphasic IO/H2O solvent system has been attributed to the solvation of phenyl ring of acetophenone by non-polar isooctane, which facilitates the interaction with the catalyst surface via carbonyl group and leads to the preferential reduction to 1-phnenylethanol.
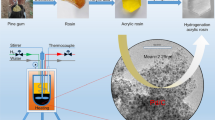
Graphical Abstract
Liquid phase hydrogenation of acetophenone was studied over ruthenium catalysts (1–4 wt% Ru) supported on diamine group functionalized gel-type polymer (FCN).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Catalytic hydrogenation of aromatic ketones to phenyl alcohols constitutes an important aspect of organic synthesis, affording key intermediates in the production of fine chemicals and drugs. The hydrogenation of acetophenone (ACT) is a typical example of this type of reactions. Selective hydrogenation of ketones can be achieved with homogeneous catalysts and most of hydride reduction of ACT exclusively gives 1-phenylethanol, as described in recently published reviews [1–3]. Unfortunately, the problems in products separation, recovery and reuse of catalysts create demand for heterogeneous catalysts. A number of studies have already been directed to hydrogenation of ACT using heterogeneous Pt, Pd, Rh, and Ru catalysts in reactions carried out under liquid- or gas-phase conditions [4–13]. Selective hydrogenation of ACT to 1-phenylethanol (PE) is complicated by the fact that different side reactions may take place. In consequence, achieving high selectivity to 1-phenylethanol, the desired product, is a challenging issue. On Pt and Rh catalysts the comparable rates of C=O and phenyl ring hydrogenations result in a mixture of various products [6, 7, 9, 10]. An exception is Pt/TiO2 catalyst which exhibit high and selective ability for C=O activation due to the presence of active centers formed as the result of strong metal support interactions [14–16]. Palladium supported catalysts, besides being highly reactive toward C=O hydrogenation to C–OH, they catalyze the hydrogenolysis of C–OH to give ethylbenzene as a final product [13, 17–20]. Ruthenium supported catalysts are also tested in hydrogenation of ACT because of their high ability to hydrogenate carbonyl group in the vicinity of conjugated and isolated double bonds [11, 12, 21, 22]. Indeed, in the case of citral and cinamaldehyde a highly selective hydrogenation to the corresponding unsaturated alcohols is observed [11]. However, when Ru catalysts are used in hydrogenation of aromatic aldehydes or ketones, such as acetophenone, they exhibit comparable reactivity toward hydrogenation of carbonyl group and aromatic ring of ACT [12, 21, 22]. In hydrogenation of ACT on Ru-catalysts two alternative reaction pathways are observed (Scheme 1) [11]. One involves hydrogenation of C=O group to give C–OH in 1-phenylethanol (PE), the other leads to hydrogenation of aromatic ring of ACT to form cyclohexyl methyl ketone (CMK). Both products, PE and CMK, undergo further but slower hydrogenation to 1-cyclohexylethanol (CE) as a final product. Other products, such as ethylbenzene, benzene, cyclohexene and cyclohexane are also formed, but in much smaller quantities [12]. In order to enhance selectivity of C=O hydrogenation an appropriate support or modification of Ru-catalysts with metal additives are needed. Results obtained by Cerveny et al. [12] and by Wismeijer et al. [22] show that among Ru-catalysts supported on Al2O3, SiO2, C and TiO2, the best efficiency in selective formation of PE is observed for Ru/TiO2 catalyst. The effect is assigned to the possible activation of the carbonyl double bond by Ti3+ cations, formed on the TiO2 support [22]. Also, the promotion of silica supported Ru catalysts with Cr ions enhances the selective reduction of C=O, but the overall activity decreases [23]. The selectivity increase is attributed to the strong interaction of the oxygen atom of C=O group with the Cr ions. In the present work hydrogenation of ACT is studied on Ru-supported catalysts using biphasic solvent system composed of less polar isooctane and polar water (IO/H2O). According to the literature, in biphasic IO/H2O solvent system a variety of catalytic reductions can effectively proceed under mild conditions (ca. 50 °C, atmospheric pressure of hydrogen) and their selectivity can be modified [24–27]. Thus, Marques et al. [24] demonstrate that hydrogenation of propiophenone on Pd/C catalyst in conventional ethanol solvent produces propylbenzene as the final product, while in the biphasic IO/H2O system propiophenone is almost selectively reduced to 1-phenyl-1-propanol [24]. In contrast, on Pt/C catalyst and in IO/H2O system, the hydrogenation of acetophenone and propiophenone proceeds towards the full reduction products, ethyl benzene and ethyl cyclohexane, respectively [25, 26]. Biphasic IO/H2O system is also successfully used for enantioselective hydrogenation of ACT in the presence of cinchona-modified Pt/C catalyst [27]. Moreover, our preliminary catalytic data demonstrate the advantage of biphasic IO/H2O solvent system in hydrogenation of ACT in the presence of Ru-catalysts supported on methacrylate-styrene gel-type resin (FCN) (Scheme 2). The FCN polymer is functionalized with C=O, –NH, and –NH2 groups and is used for the preparation of palladium catalysts with finely dispersed Pd-nanoparticles [28, 29]. Our previous results show that functional groups of FCN resin, and in particular the N-containing ones participate in the bonding of Pd, Ru-species via filling metal coordination sphere [28–30]. Functional groups may also affect the electron properties of active centers and in consequence catalytic properties of studied Ru/FCN samples.
Homogeneous Ru(III) complexes with nitrogen and oxygen-containing ligands like diamine, diamino alcohols, amine carboxylic etc. are capable of catalyzing various reactions, oxidation, hydrogenation and hydrogen transfer reactions. Traditionally, insoluble polymer resins such as divinylbenzene, cross-linked polystyrene have been studied as supports for heterogenization of such catalytically active complexes. Other, but more convenient way to prepare polymer-anchored catalytically active metal species is the use of polymers that are functionalized with appropriate coordinating ligands, such as β-diketones (O,O), dipyridyl amine (N,N), diphosphine (P,P) etc. For example, Ru immobilized on poly(4-vinylpyridine) is reported to be an efficient catalyst for selective hydrogenation of aromatic ring of quinoline [31]. Ru supported on potassium methacryloyl ethylene sulphonate resin is found to be efficient in partial hydrogenation of benzene [32], and Ru immobilized by chloromethylated styrene-divinyl benzene copolymer functionalized by glycine is effective for hydrogenation of nitrobenzene [33]. This method, based on the usage of polymer functionalized with coordinating ligands is also applied in the present work.
Here, the role of biphasic IO/H2O solvents system in hydrogenation of ACT is studied using Ru/FCN catalysts of various Ru content. For comparison, the reaction is also studied in the presence of γ-Al2O3 supported Ru.
2 Experimental
2.1 Materials
Acetophenone is purchased from Sigma-Aldrich, 1-phenylethanol, 1-cyclohexylethanol, cyclohexyl methyl ketone are from Fluka, and isooctane is from POCH. All chemicals are used as purchased.
2.2 Preparation of Catalysts
The FCN resin (3% crosslinking degree) (77 wt% styrene, 2.78 wt% N content) [28] swells very well in THF and this solvent is used as a medium during the preparation of Ru-composites (1, 2 and 4 wt% Ru) according to the previously described procedure [30]. Thus, FCN resin pre-swollen in THF is treated with appropriate amount of aqueous solution of RuCl3 under slow mixing up to the complete incorporation of ruthenium species. After washing and drying in air, the as-received composites are reduced by NaBH4 solution in THF:CH3OH (9:1 volume ratio) using 10-fold excess. The resulting black Ru-polymer beads are washed with THF/acetone several times and dried in air. 2%Ru/Al2O3 catalyst (γ-Al2O3, BET surface area 155 m2/g, particles ca. 100 μm) is prepared by impregnation method using aqueous solution of RuCl3, followed by reduction with NaBH4 [30].
2.3 Characterization Methods
The reduced Ru/FCN catalysts are characterized by XRD, SEM and STEM techniques. X-ray diffraction patterns (XRD) are obtained with a Siemens D5005 diffractometer using Cu Kα radiation. Morphology of catalysts is studied by means of Field Emission Scanning Electron Microscope JEOL JSM—7500 F equipped with the X-ray energy dispersive (EDS) system. The secondary electron detector provides SEI images and back scattered electron detector provides BSE (COMPO) micrographs. K575X Turbo Sputter Coater is used for coating the specimens with chromium (deposited film thickness—20 nm). STEM studies are performed on FEI Tecnai G2 transmission electron microscope at 200 kV equipped with EDAX EDX and HAADF/STEM detectors.
2.4 Hydrogenation Experiments
Hydrogenation experiments are carried out in an agitated batch glass reactor at constant atmospheric pressure of hydrogen and temperature of 40 °C [30]. A biphasic solvent system, composed of isooctane and water (2× distilled) in 1:1 volume ratio is used. In a typical hydrogenation test the concentration of ACT is 0.137 mol/dm3 (referred to isooctane phase) and the concentration of catalyst is 10 g/dm3, referred to biphasic IO/H2O solvent system. Before the hydrogenation experiment the catalyst, wetted with water, is activated inside the reactor in a flow of hydrogen for 45 min at 20 °C and 45 min at the temperature of reaction (40 °C). For comparison, in hydrogenation tests isooctane or ethanol are used as a single solvent. In the course of catalytic runs the samples of reaction mixture (organic phase) are periodically withdrawn and analyzed by GC method [30]. The contents of ACT, PE, CMK, and CE are determined by comparison with calibration curves. From the data of GC analysis acetophenone conversion (C, %) and selectivity [S(i)] to products, 1-phenylethanol [S(PE)], cyclohexyl methyl ketone [S(CMK)] and 1-cyclohexylethanol [S(CE)] are calculated from the equations:
where, n 0(ACT) and n t(ACT) are the moles of acetophenone initially present in the reactor and the moles of ACT remaining at time t, respectively; n t(N i ) are number of moles of individual reaction products, PE, CMK, CE, at reaction time t.
2.5 Determination of the Partitioning of Reaction Mixture
In two-phase isooctane-water system the distribution of all the reagents (ACT, PE, CMK, and CE) in both solvent phases is determined by GC method. The known amounts of particular reagents (137 × 10−5 mol of ACT, 199 × 10−5 mol of PE, 34 × 10−5 mol of CMK, and 43 × 10−5 mol of CE) are added into 20 cm3 of IO/H2O (1:1 by volume). The whole lot is vigorously mixed with a mechanical shaker for 45 min. The samples of liquids from both phases are withdrawn immediately after the completion of shaking, and after 5, 15, 30, and 60 min. The concentrations of ACT, PE, CMK, and CE in both isooctane and aqueous phases are determined using GC method. The equilibrium concentrations of ACT, PE, CMK, and CE are established just after stopping the mixing. The isooctane phase (top layer) contains 92.7% of ACT, 55.6% of PE, 100% of CMK, and 93.0% of CE. From these data the distribution coefficients: 1.08 for ACT and CE, and 1.80 for PE are calculated and used for the determination of the total concentrations of reagents during the catalytic test on the basis of GC analysis of organic phase only.
3 Results and Discussion
3.1 Characterization of Ru/FCN Catalysts
Preparation and characterization of Ru/FCN composites used in the present study in the capacity of catalysts is described in detail in our previous work [30]. Briefly, the results obtained by number of techniques (FT-IR, XPS, DSC, SEM, EDS) show that functional groups of FCN polymer, in particular the amine groups –NH, and –NH2, participate in the coordination of Ru(III) ions. The coordination sphere of ruthenium trapped within the FCN matrix contains both Cl and N-ligands in various proportions depending on Ru loading in the polymer. Introductory catalytic experiments show no hydrogenation of ACT when as-prepared Ru/FCN composites are tested. In the presence of NaBH4 reduced 2%Ru/FCN catalyst hydrogenation of ACT is effective, however only when reaction is performed in biphasic IO/H2O solvent system. On the other hand, in ethanol, THF, or isooctane as the only solvent the reaction is very slow, practically no measurable under conditions of catalytic tests [30]. Therefore present studies are undertaken to shed more light on the advantageous role of biphasic IO/H2O solvent system in the hydrogenation of ACT. Here, reaction is studied in the presence of reduced Ru/FCN catalysts and for the comparison 2%Ru/Al2O3 catalyst is tested.
Ru-particles in reduced Ru/FCN catalysts are characterized by XRD and electron microscopy (SEM and TEM) techniques. The SEM studies are carried out in SEI and BSE (COMPO) modes. SEI (secondary electron imaging) is appropriate for studying the morphology of catalyst. The BSE (COMPO) registration mode provides information about the distribution of Ru in the catalyst because the brightness observed in BSE images is strongly related to atomic number. BSE (COMPO) images of reduced Ru/FCN composites with 1, 2 and 4 wt% Ru are displayed in Fig. 1. A number of irregular “bright” spots of various shapes and sizes randomly oriented and distributed throughout the polymer beads can be observed in all catalysts. However, no reflections associated with the particles of crystalline Ru appear in their XRD diffraction pattern (Fig. 2a). Thus, irregularly shaped “bright” spots observed in BSE images may be considered to be associated with the presence of aggregated nano-particles of ruthenium. In order to clarify this supposition, the “areas of bright spots” are examined at higher magnification and the representative SEI and BSE images are shown in Fig. 3. The SEI image (Fig. 3a) shows that polymer is composed of almost spherical grains of similar shape and size within the range 100–150 nm. The BSE (COMPO) image (Fig. 3b) reveals that, among spherical polymer grains, agglomerates of much brighter grains exist, which indicates that the concerned areas are substantially enriched in ruthenium.
The nature of Ru particles in catalysts of various Ru-content is characterized by the STEM micrographs in which ruthenium particles appear as bright spots and/or areas against darker background originating from the polymer matrix (Figs. 4 and 5). At the lowest Ru loading in polymer, 1%Ru/FCN (Fig. 4), no separated particles of Ru can be distinguished but the whole polymer matrix appears much brighter. Upon increasing Ru loading up to 2 wt% Ru, apart from “bright areas of polymer” the separated bright spots can be observed showing that ruthenium aggregates start to form. EDS analysis of Ru carried out in marked boxes (1, 2, 3) indicates the presence of ruthenium even in the areas apparently almost free of bright spots. However, electron diffraction shows no evidence of any crystalline Ru phase in both 1%Ru/FCN and 2%Ru/FCN catalysts, which points to the presence of Ru-containing nano-clusters.
STEM image of catalyst with high Ru loading, 4%Ru/FCN, shows a qualitative difference (Fig. 5). Clearly observable bright spots of metallic ruthenium are visible (Fig. 5a) and the electron diffraction pattern originating from crystalline ruthenium (Fig. 5b) is obtained. Although the size of such Ru-crystalline particles is within wide range, their sizes do not exceed few nanometers. Thus, upon increasing ruthenium loading in the FCN polymer the nature of Ru-particles is changed from very highly dispersed Ru-nanoclusters to nanocrystallites of few nanometers in size. This may be explained taking into account that amine –NH, and –NH2 groups of FCN resin (Scheme 2) participate in the immobilization of Ru(III) ions. In the coordination sphere of Ru(III)-species immobilized by FCN resin both Cl and N-ligands are observed, however in various proportions depending on the Ru content introduced into polymer [30]. At low loading of Ru (1 and 2 wt%) the contribution of N-groups of polymer in the coordination sphere of Ru-species is high and it decreases as the loading of Ru grows to 4 wt%. In catalysts with low Ru content (1 and 2 wt%) highly dispersed Ru-nanoclusters are observed showing that high contribution of N-groups of polymer in the Ru-species favors the formation of highly dispersed Ru catalysts. On the other hand, functional groups may affect the electron properties of Ru-centers and in consequence catalytic properties of Ru/FCN samples. The influence of functional groups, however, would be to some extent determined by the size of Ru-particles. It may be expected that the higher Ru dispersion, the more important effect of functional groups of polymer matrix.
The electron microscopic images of 2%Ru/Al2O3 catalyst are displayed in Fig. 6. The “white spots” of Ru particles of various shapes and size are observed. Only a few isolated “white spots” appear whereas most of them form aggregates of size within the range 10–50 nm. In the XRD diffraction pattern of 2%Ru/Al2O3 the broad and low intense reflexes of crystalline Ru located at 38.1° and 41.1° appear (Fig. 2b) indicating the presence Ru particles of size of few nanometers. Thus, the “white spots” observed in the SEM micrograph are the aggregated nanoparticles of metallic Ru.
3.2 Catalytic Experiments
3.2.1 Hydrogenation of ACT in the Presence of 2%Ru/Al2O3 Reference Catalyst
The first part of present work concentrates on ACT hydrogenation in the presence of 2%Ru/Al2O3 catalyst. Reaction is studied in ethanol and isooctane as the only solvents and then in biphasic IO/H2O solvent system. In all experiments the same catalyst concentration (10 g/dm3) is used. At this amount of catalyst no hydrogenation of ACT is observed in isooctane solvent whereas reaction is effective in ethanol and IO/H2O solvent system. The conversions of ACT obtained in ethanol and in biphasic IO/H2O solvents are compared in Fig. 7a, and the selectivities to PE, CMK and CE are reported in Fig. 7b. In ethanol solvent, the hydrogenation of ACT is effective, but the reaction proceeds at a very low rate and about 20% ACT conversion is attained after ca. 320 min of reaction. The rate of ACT hydrogenation distinctly grows in biphasic IO/H2O solvent system and after comparable reaction time (ca 300 min) almost 70% of ACT conversion is attained. Furthermore, induction periods appear, especially clearly observable in IO/H2O solvents system. It should be stressed that in the case of ruthenium catalysts induction periods are quite common, especially in reactions carried out at low hydrogen pressure in nonaqueous media. At high hydrogen pressures, induction periods rarely occur, regardless of solvent used [34].
A substantial difference can be seen regarding the selectivity of ACT hydrogenation (Fig. 7b). In reaction media, ethanol and IO/H2O solvent system, ACT is hydrogenated via both reaction pathways. The reduction of C=O to form phenylethanol (PE) as well as the hydrogenation of aromatic ring yielding cyclohexyl methyl ketone (CMK) take place (Scheme 1). Moreover, 1-cyclohexyl ethanol is also formed. In both solvent systems, the reduction of C=O predominates over the hydrogenation of aromatic ring, and this predomination is much higher in biphasic IO/H2O solvent system. In ethanol, selectivity to PE attain ca. 60% and that to CMK is ca. 40% (Fig. 7b). Owing to low rate of ACT conversion in ethanol, the data are obtained at ca. 40% conversion of ACT only. This selectivity pattern in ethanol solvent is similar to that reported by Cerveny et al. [12] who demonstrated comparable selectivities to PE and CMK on 5%Ru/Al2O3 catalyst.
On the other hand, in IO/H2O solvent system the rate of ACT conversion is distinctly higher and the raction is studied up to almost complete conversion of ACT. In such biphasic IO/H2O solvent system selectivity to PE is much higher compared to that of CMK. Selectivity to PE as high as ca. 80% is reached at the beginning of the reaction. However, as the reaction progresses the selectivity to PE slowly but continuously decreases whereas the selectivity to 1-cyclohexylethanol (CE) grows. This may suggest that as PE is formed, it is consumed via hydrogenation of aromatic ring to form 1-cyclohexylethanol (CE). As Fig. 7b shows, selectivity to CMK is only 20% and practically does not change up to the almost complete conversion of ACT. This indicates that only 20% from the reacted ACT is transformed via aromatic ring hydrogenation whereas 80% is reacted via C=O reduction. Thus, in biphasic IO/H2O system the rate of ACT hydrogenation and the selectivity to PE are higher compared to the reaction in ethanol. This reveals promoting effect of biphasic IO/H2O solvent system in activity of 2%Ru/Al2O3 and in selective reduction of C=O in ACT to give 1-phenylethanol (PE).
3.2.2 Hydrogenation of ACT in the Presence of Ru/FCN Catalysts
The conversion of ACT in the presence of Ru/FCN catalysts is displayed in Fig. 8a. Reaction is studied at catalyst concentration of 10 g/dm3. Under such conditions the activity of 1%Ru/FCN is very low and only 2% of ACT conversion is achieved after 300 min of reaction time (data not shown). The hydrogenation of ACT over 2%Ru/FCN and 4%Ru/FCN catalysts is much more effective, the activity of 4%Ru/FCN being distinctly higher than that of 2%Ru/FCN. A different “shape” of conversion plots and, in particular, a pronounced induction period observed in the presence of 4%Ru/FCN makes difficult calculation and comparison of reaction rates. Selectivity patterns (Fig. 8b) shows that on both Ru/FCN catalysts ACT is preferentially transformed via hydrogenation of C=O to give unsaturated alcohol, PE. The selectivity to CMK is only ca. 11–12% on 2%Ru/FCN and slightly lower 6–7% on 4%Ru/FCN catalyst. Similarly to 2%Ru/Al2O3, the selectivity to CMK is practically stable up to almost complete conversion of ACT. As the reaction progresses the selectivity to PE slowly decreases whereas that of CE grows. Thus, polymer supported Ru/FCN catalysts show clear preference for catalyzing C=O reduction rather than hydrogenation of phenyl ring, and this preference is somewhat stronger for the 4%Ru/FCN catalyst. This may be associated with the differences in the nature of Ru particles in both catalysts. In 2%Ru/FCN catalyst ruthenium exists mainly in the form of well dispersed nano-clusters, while in 4%Ru/FCN catalyst, Ru particles, although still not larger than few nanometers, exhibit crystalline character. Moreover, in the presence of both 2%Ru/FCN and 4%Ru/FCN catalysts, predomination of C=O reduction over aromatic ring hydrogenation is higher compared to that of 2%Ru/Al2O3.
The effect of 4%Ru/FCN catalyst concentration (7.5–12.5 g/dm3) is shown in Fig. 9. Within the whole range of catalyst concentration an induction period is observed making the calculation of hydrogenation rate complex (Fig. 9a). Nevertheless, the rate of ACT conversion increases as the concentration of 4%Ru/FCN catalyst grows up to 10 g/dm3. At higher concentration, 12.5 g/dm3, the rate decreases, which is most likely associated with limited supply of hydrogen to the catalyst surface. Therefore in all experiments the concentration of catalyst 10 g/dm3 is used. The concentration of 4%Ru/FCN does not affect the selectivity pattern of ACT hydrogenation (Fig. 9b) because PE is formed as the major product. The selectivity to CMK, product of aromatic ring hydrogenation is very low, ca. 6–7% and practically does not change during the hydrogenation experiment.
The hydrogenation is also studied using 2-times lower initial ACT concentration (Fig. 10). The induction periods are observed in both solutions but induction period is longer at lower initial ACT concentration. However, both conversion curves vs. reaction time are parallel (Fig. 10), which shows that the rates of ACT conversion do not depend on initial ACT concentration. Moreover, initial ACT concentration does not influence substantially the selectivity of C=O reduction, which is still high regardless of initial ACT concentration.
3.3 Recovery and Reuse
A frequently encountered problem of polymer supported catalysts is the decrease of their activity due to the leaching of metal particles under catalytic test. In order to determine whether Ru/FCN are stable under reaction conditions the 2%Ru/FCN catalyst is recovered and reused twice. After each hydrogenation test, the catalyst is separated by filtration, washed with acetone, dried in air, and then subjected to the next catalytic cycle. Fresh ACT solution is added and the same hydrogenation conditions are applied. The decrease of ACT content (mol %) against reaction time in the first and the third catalytic runs are practically the same (Fig. 11a). The selectivity to PE as well as to CMK (Fig. 11b) after 3 reaction cycles are the same as those of fresh catalyst showing that high selectivity towards carbonyl group in ACT remains unchanged.
This set of experiments proves stable activity of 2%Ru/FCN in recycling hydrogenation experiments which most likely results from the multidentate character of FCN resins (Scheme 2). As mentioned before, in FCN matrix chelating functional groups (–NH, –NH2, C=O) appear, able to fill coordination sites of ruthenium making the metal centre strongly bound to the support. This is in agreement with the literature data [35] showing that leaching of the metal from the polymer can be reduced significantly by using polymer functionalized by chelating ligands.
3.4 The Role of the Bi-phasic Solvent System
In liquid phase catalytic reactions the type of solvent frequently influences the rate and selectivity of reaction. The effect has usually a complex nature and may be related to reactant-solvent, product-solvent and/or catalyst-solvent interactions [34, 36]. Often, the solvent effect is rationalized by correlating the reaction rates and product distribution with solvent polarity or dielectric constant [13, 19]. For instance, the activity of Pd/AlPO4 in hydrogenation of ACT in various alcohols decreases when increasing the alcohol dielectric constant [18]. Similarly, on Raney Ni catalyst the ACT hydrogenation is faster in cyclohexane than in alcohol solvents [37]. Moreover, apart from polarity of solvents, the nature of support in Pd catalysts is found to affect the rate and selectivity of ACT hydrogenation [38]. Solvent effect can be manifested among others, in interactions with the reactants molecules via the solvation effect. From the experiments of competitive hydrogenation of non-polar and polar substrates, a general conclusion has been formulated, indicating that a polar solvent enhances adsorption of a non-polar reactant at the catalyst surface while a non-polar solvent promotes adsorption of a polar reactant [34, 36]. Due to such solvent–substrate solvation effect, the selectivity and the type of product formed may change significantly. In reactants possessing polar groups, interaction with the solvent can affect different fragments of the molecules, depending on the solvent polarity. The hydrogenation of p-phenylphenol, which contains less polar unsubstituted phenyl ring and more polar OH-substituted phenyl rings, is a good illustration [34]. In the polar water–acetic acid solvents, p-cyclohexylphenol is the major product, because OH-substituted phenolic ring is more strongly solvated and thus less readily adsorbed on the catalyst surface. Conversely, more effective solvation of less polar unsubstituted phenyl ring by less polar cyclohexane solvent resulted in preferential adsorption of OH-substituted phenolic ring and preferential formation of phenylcyclohexanol.
In biphasic IO/H2O system the reduction of C=O predominates over the hydrogenation of aromatic ring in the ACT substrate. Moreover, the reaction in biphasic IO/H2O system is faster than in ethanol.
While trying to find a rationale for the observed phenomenon, one should recall that in biphasic IO/H2O solvent system the overwhelming majority of ACT is contained in less polar isooctane. On the other hand, the primary function of the water is believed to be making the surrounding of Ru centers more hydrophilic, similarly to what is postulated for benzene–water system used for hydrogenation of Ru-supported catalysts [39, 40]. Under such conditions the ACT molecule assumes orientation in which its less polar part, i.e. phenyl ring, is directed towards non-polar isooctane, while the more polar carbonyl group is exposed toward Ru centers. Additionally, the enhanced hydrophilicity of catalyst surface may facilitate interaction of Ru-centers with the lone pair of the electrons on the carbonyl oxygen, which polarizes the C=O bond, and hence promotes its hydrogenation. For the same reason, in biphasic IO/H2O system, the adsorption of ACT molecule at the catalyst surface in a flat configuration, involving simultaneous adsorption of aromatic ring and carbonyl group, is much less likely. Thus, the observed effect of biphasic IO/H2O system can be explained by its role in controlling the orientation of ACT molecule and enhancing the hydrophilicity of the catalyst surface. This favors catalyst–ACT interaction via carbonyl group and preferential reduction of ACT to PE.
Comparison of the catalytic performance of Ru/FCN and Ru/Al2O3 catalysts shows that the preference for the selective C=O reduction is more pronounced in the presence of FCN polymer–supported Ru. This advantageous effect points to the crucial role of the polymer support. It is likely that the polymer chains surrounding the Ru particles contribute to a desired steric orientation of the ACT reactant.
4 Conclusions
Ruthenium catalysts supported on amine functionalized methacrylate-styrene FCN resin prove very active and highly selective in the hydrogenation of carbonyl group in acetophenone. In the presence of 4%Ru/FCN catalyst 1-phenylethanol is formed with ca. 80% selectivity. The essential feature of the catalytic set-up is the use of a biphasic isooctane/water solvent system. An important aspect of reaction carried out in the biphasic isooctane/water solvent is that acetophenone becomes transformed preferentially via hydrogenation of C=O group rather than hydrogenation of aromatic ring. The role of biphasic isooctane/water solvent system has been attributed to the solvation of acetophenone phenyl ring by non-polar isooctane, which enhances appropriate orientation of the reactant and facilitates adsorption and catalytic transformation of polar carbonyl group at the surface of the catalyst. Superior selectivity to 1-phenylethanol over polymer-supported ruthenium catalyst with respect to the reference Ru/Al2O3 catalyst is tentatively assigned to steric effects induced by the polymer chains which enhance favorable orientation of acetophenone molecule towards Ru active sites.
References
Blaser H-U, Malan Ch, Pugin B, Spindler F, Steiner H, Studer M (2003) Adv Synth Catal 345:103
Wang Ch, Wu X, Xiao J (2008) Chem Asian J 3:1750
Noyori R, Ohkuma T (2001) Angew Chem Int Ed 40:40
Rajashekharam MV, Bergault I, Fouilloux P, Schweich D, Delmas H, Chaudhari RV (1999) Catal Today 48:83
Malyala RV, Rode CV, Arai M, Hedge SG, Chaudhari RV (2000) Appl Catal A 193:71
Bergault I, Fouilloux P, Joly-Vuillemin C, Delmas H (1998) J Catal 175:328
Bergault I, Joly-Vuillemin C, Fouilloux P, Delmas H (1999) Catal Today 48:161
Bertero NM, Apesteguia CR, Marchi AJ (2008) Appl Catal A 349:100
Chen CS, Chen HW, Cheng WH (2003) Appl Catal A 248:117
Santori GF, Moglioni AG, Vetere V, Moltrasio Iglesias GY, Casella ML, Ferretti OA (2004) Appl Catal A 269:215
Kluson P, Cerveny L (1995) Appl Catal A 128:13
Cerveny L, Dobrovolna Z, Belohlav Z, Kluson P (1996) Collect Czechoslov Chem Commun 61:764
Drelinkiewicz A, Waksmundzka A, Makowski W, Sobczak JW, Król A, Zięba A (2004) Catal Lett 94:143
Lin SD, Sanders DK, Vannice MA (1994) Appl Catal A 113:59
Vannice MA (1992) Catal Today 12:255
Vannice MA (1997) Top Catal 4:241
Meschke RW, Hartung WH (1960) J Org Chem 25:137
Aramendia MA, Borau V, Gomez JF, Herrera A, Jimenez C, Marinas JM (1993) J Catal 140:335
Aramendia MA, Borau V, Jimenez C, Marinas JM, Sempere ME, Urbano P (1988) Appl Catal 43:41
Tundo P, Zinovyev S, Perosa A (2000) J Catal 196:330
Kluson P, Cerveny L (1996) J Mol Catal A 108:107
Wismeijer AA, Kieboom APG, van Bekkum H (1985) React Kinet Catal Lett 29:311
Casagrande M, Storaro L, Talon A, Lenarda M, Frattini R, Rodriguez-Castellon E, Maireles-Torres P (2002) J Mol Catal A 188:133
Marques CA, Selva M, Tundo P (1995) J Org Chem 60:2430
Tundo P, Perosa A, Zinovyev S (2003) J Mol Catal A 204–205:747
Perosa A, Selva M, Tundo P (1999) J Org Chem 64:3934
Perosa A, Tundo P, Selva M (2002) J Mol Catal A 180:169
Drelinkiewicz A, Stanuch W, Knapik A, Ghanem A, Kosydar R, Bukowska A, Bukowski W (2009) J Mol Catal A 300:8
Drelinkiewicz A, Knapik A, Stanuch W, Sobczak J, Bukowska A, Bukowski W (2008) React Funct Polym 68:1650
Duraczynska D, Drelinkiewicz A, Serwicka EM, Rutkowska-Zbik D, Bielańska E, Socha R, Bukowska A, Bukowski W (2010) React Funct Polym 70:382
Sanchez-Delago RA, Machalaba N, Ng-a-qui N (2007) Catal Commun 8:2115
Hronec M, Cvengrosova Z, Kralik M, Palma G, Corain B (1996) J Mol Catal A 105:25
Patel DR, Dalal MK, Ram RN (1996) J Mol Catal A 109:141
Rylander PN (1967) Catalytic hydrogenation over platinum metals. Academic Press, New York, p 238
Michalska Z, Strzelec K (2001) J Mol Catal A 177:89
Singh UK, Vannice MA (2001) Appl Catal A 213:1
Masson J, Cividino P, Bonier JM, Fouilloux P (1991) Stud Surf Sci Catal 245
Bejblova M, Zamostny P, Cerveny L, Cejka J (2003) Collect Czech Chem Commun 68:1969
Struijk J, d’Angremond M, Lucas de Regt WJM, Scholten JJF (1992) Appl Catal A 83:263
Milone C, Neri G, Donato A, Musolino MG, Mercadante L (1996) J Catal 159:253
Acknowledgements
D. D. acknowledges the financial support from the POL-POST DOC II (project D037/H03/2006) and grant NN204 249034 (project 2490/B/H03/2008/34). The authors thank Prof. W. Bukowski and dr A. Bukowska from Rzeszow University of Technology for preparation of polymeric support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Duraczyńska, D., Drelinkiewicz, A., Bielańska, E. et al. Hydrogenation of Acetophenone in the Presence of Ru Catalysts Supported on Amine Groups Functionalized Polymer. Catal Lett 141, 83–94 (2011). https://doi.org/10.1007/s10562-010-0448-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0448-3