Abstract
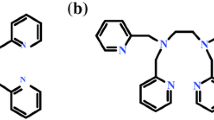
Two manganese-containing catalysts have been employed in the oxidation with hydrogen peroxide of two reactive alcohols (1-phenylethanol and glycerol): soluble catalyst [LMn(μ-O)3MnL](PF6)2 (1a) and heterogenized catalyst [LMn(μ-O)3MnL]2[SiW12O40] (1b) (L is 1,4,7-trimethyl-1,4,7-triazacyclononane, TMTACN). Oxidation of 1-phenylethanol catalyzed by 1a in acetonitrile solution proceeds at room temperature in the presence of a small amount of oxalic acid; the turnover number attains 15,000 after 3 h. It has been proposed on the basis of the kinetic study that an oxidizing species is a manganyl species containing fragment “Mn=O” rather that hydroxyl radical. This species reacts competitively with the alcohol, acetonitrile and hydrogen peroxide. In the case of 1b dependences of the initial rates of acetophenone accumulation on concentration of the alcohol and amount of 1b have plateau. Both homogeneous and heterogeneous catalysts are efficient in the oxidation of glycerol to produce dihydroxyacetone (DHA) as the main product. The oxidation catalyzed by 1a is one of the first examples of the glycerol oxidation by a catalytic homogeneous system. The yield of valuable products attained 45%. The oxidation of DHA in the absence of glycerol afforded mainly glycolic acid in yield 60% based on the starting DHA. The oxidation on 1b represents the first example of the glycerol transformation catalyzed by a heterogenized metal complex. Under certain conditions yields of products of deeper oxidation (glyceric, glycolic and hydroxypyruvic acids) are somewhat higher than the yield of dihydroxyacetone. Special experiments demonstrated that no leaching of active species occurs from catalyst 1b to the solution and that this catalyst can be re-used at least four times without substantial loss of activity.
Graphical Abstract
Manganese-containing complexes are very efficient catalysts in the oxidation of reactive alcohols (1-phenylethanol and glycerol) with H2O2: soluble [LMn(O)3MnL](PF6)2 and heterogenized [LMn(O)3MnL]2[SiW12O40] (L is 1,4,7-trimethyl-1,4,7-triazacyclononane).

















Similar content being viewed by others
References
Strukul G (ed) (1992) Catalytic oxidations with hydrogen peroxide as oxidant. Kluwer Academic Publishers, Dordrecht
Muzart J (2003) Tetrahedron 59:5789–5816
Marko IE, Giles PR, Tsukazaki M, Gautier A, Dumeunier R, Doda K, Philippart F, Chellé-Regnault I, Mutonkole J-L, Brown SM, Urch CJ (2004) Aerobic, metal-catalyzed oxidation of alcohols. In: Beller M, Bolm C (eds) Transition metals for organic synthesis, vol 2, 2nd edn. Wiley–VCH: Weinheim/New York, pp 437–478
Mallat T, Baiker A (2004) Chem Rev 104:3037–3058
Tojo G, Fernandez M (2006) Oxidation of alcohols to aldehydes and ketones. Springer Science, Business Media, Inc, New York
Seki T, Baiker A (2009) Chem Rev 109:2409–2454
Prati L, Porta F (2005) Appl Catal A: General 291:199–203
Korovchenko P, Donze C, Gallezot P, Besson M (2007) Catal Today 121:13–21
Figiel PJ, Kirillov AM, Karabach YY, Kopylovich MN, Pombeiro AJL (2009) J Mol Catal A: Chem 305:178–182
Jiang N, Vinci D, Liotta CL, Eckert CA, Ragauskas AJ (2008) Ind Eng Chem Res 47:627–631
Haider P, Grunwaldt J-D, Baiker A (2009) Catal Today 141:349–354
Figiel PJ, Sobczak JM (2009) J Catal 263:167–172
Pérez BM, Hartung J (2009) Tetrahedron Lett 50:960–962
Villa A, Janjic N, Spontoni P, Wang D, Su DS, Prati L (2009) Appl Catal A: General 364:221–228
Lecomte V, Bolm C (2005) Adv Synth Catal 347:1666–1672
Shi F, Tse MK, Pohl M-M, Radnik J, Brückner A, Zhang S, Beller M (2008) J Mol Catal A: Chem 292:28–35
Hida T, Nogusa H (2009) Tetrahedron 65:270–274
Ye Z, Fu Z, Zhong S, Xie F, Zhou X, Liu F, Yin D (2009) J Catal 261:110–115
Lounis Z, Riahi A, Djafri F, Muzart J (2006) Appl Catal A: General 309:270–272
Tarlani A, Riahi A, Abedini M, Amini MM, Muzart J (2006) Appl Catal A: General 315:150–152
Della Pina C, Falletta E, Rossi M (2008) J Catal 260:384–386
Haider P, Kimmerle B, Krumeich F, Kleist S, Grunwaldt J-D, Baiker A (2008) Catal Lett 125:169–176
Xu S, Yan X, Yao Y, He X, Chen Y (2009) Shiyou Huagong (Petrochemical Technology) 38:193–196
Dimitratos N, Lopez-Sanchez JA, Morgan D, Carley AF, Tiruvalam R, Kiely CJ, Bethell D, Hutchings GJ (2009) Phys Chem Chem Phys 11:5142–5153
Van Gerpen J (2005) Fuel Process Technol 86:1097–1107
da Silva CRB, Gonçalves VLC, Lachter ER, Mota CJA (2009) J Braz Chem Soc 20:201–204
de Rezende SM, de Castro Reis M, Reid MG, Silva PL Jr, Coutinho FMB, Gil RASS, Lachter ER (2008) Appl Catal A: General 349:198–203
Sels B, D’Hondt E, Jacobs P (2007) Catalytic transformation of glycerol. In: Centi G, van Santen RA (eds) Catalysis for renewables. Wiley-VCH Verlag, Weinheim, pp 223–255
Corma A, Iborra S, Velty A (2007) Chem Rev 107:2411–2502
Zhou C-H, Beltramini JN, Fan Y-X, Lu GQ (2008) Chem Soc Rev 37:527–549
Zheng Y, Chen X, Shen Y (2008) Chem Rev 108:5253–5277
Behr A, Eilting J, Irawadi K, Leschinski J, Lindner F (2008) Green Chem 10:13–30
Pagliaro M, Rossi M (2008) The future of glycerol. New usages for a versatile raw material. RSC Publishing, Cambridge, p 128
Behr A (2008) ChemSusChem 1:653
Garcia R, Besson M, Gallezot P (1995) Appl Catal A: General 127:165–176
Carrettin S, McMorn P, Johnston P, Griffin K, Kiely CJ, Hutchings GJ (2003) Phys Chem Chem Phys 5:1329–1336
Bianchi CL, Canton P, Dimitratos N, Porta F, Prati L (2005) Catal Today 102–103:203–212
Dimitratos N, Lopez-Sanchez JA, Lennon D, Porta F, Prati L, Villa A (2006) Catal Lett 108:147–153
Ketchie WC, Murayama M, Davis RJ (2007) J Catal 250:264–273
Taarning E, Madsen AT, Marchetti JM, Egeblad K, Christensen CH (2008) Green Chem 10:408–414
Maurino V, Bedini A, Minella M, Rubertelli F, Pelizetti E, Minero C (2008) J Adv Oxid Tech 11:184–192
Pollington SD, Enache DI, Landon P, Meenakshisundaram S, Dimitratos N, Wagland A, Hutchings GJ, Stitt EH (2009) Catal Today 145:169–175
Prati L, Spontoni P, Gaiassi A (2009) Top Catal 52:288–296
Thomas JM, Hernandez-Garrido JC, Bell RG (2009) Top Catal 52:1630–1639
Liang D, Gao J, Wang J, Chen P, Hou Z, Zheng X (2009) Catal Commun 10:1586–1590
Dimitratos N, Villa A, Prati L (2009) Catal Lett 133:334–340
Rennard DC, Kruger JS, Schmidt LD (2009) ChemSusChem 2:89–98
Demirel-Gülen S, Lucas M, Claus P (2005) Catal Today 102–103:166–172
Demirel S, Kern P, Lucas M, Claus P (2007) Catal Today 122:292–300
Demirel S, Lucas M, Wärnå J, Salmi T, Murzin D, Claus P (2007) Top Catal 44:299–305
Herzing AA, Kiely CJ, Carley AF, Landon P, Hutchings GJ (2008) Science 321:1331–1335
Lopez-Sanchez JA, Dimitratos N, Miedziak P, Ntainjua E, Edwards JK, Morgan D, Carley AF, Tiruvalam R, Kiely CJ, Hutchings GJ (2008) Phys Chem Chem Phys 10:1921–1930
Dimitratos N, Lopez-Sanchez JA, Anthonykutty JM, Brett G, Carley AF, Tiruvalam RC, Herzing AA, Kiely CJ, Knight DW, Hutchings GJ (2009) Phys Chem Chem Phys 11:4952–4961
Dimitratos N, Lopez-Sanchez JA, Anthonykutty JM, Brett G, Carley AF, Taylor SH, Knight DW, Hutchings GJ (2009) Green Chem 11:1209–1216
Clejan LA, Cederbaum AI (1992) FASEB J 6:765–770
Rashba-Step J, Step E, Turro NJ, Cederbaum AI (1994) Biochemistry 33:9504–9510
Liebminger S, Siebenhofer M, Guebitz G (2009) Bioresour Technol 100:4541–4545
da Silva GP, Mack M, Contiero J (2009) Biotechnol Adv 27:30–39
Bauer R, Katsikis N, Varga S, Hekmat D (2005) Bioprocess Biosyst Eng 28:37–43
McMorn P, Roberts G, Hutchings GJ (1999) Catal Lett 63:193–197
Luque R, Budarin V, Clark JH, Macquarrie DJ (2008) Appl Catal B: Environ 82:157–162
Sankar M, Dimitratos N, Knight DW, Carley AF, Tiruvalam R, Kiely CJ, Thomas D, Hutchings GJ (2009) ChemSusChem 2:1145–1151
Laurie VF, Waterhouse AL (2006) J Agric Food Chem 54:4668–4673
Dimitratos N, Messi C, Porta F, Prati L, Villa A (2006) J Mol Catal A: Chem 256:21–28
Kimura H, Tsuto K, Wakisaka T, Kazumi Y, Inaya Y (1993) Appl Catal A: General 96:217–228
Demirel S, Lehnert K, Lucas M, Claus P (2007) Appl Catal B: Environ 70:637–643
Brandner A, Lehnert K, Bienholz A, Luca M, Claus P (2009) Top Catal 52:278–287
Brandner A, Claus P (2009) 6th World congress on oxidation catalysis, Lille, France, report 3B-552
Wörz N, Brandner A, Claus P (2010) J Phys Chem C 114:1164–1172
Shul’pin GB, Lindsay Smith JR (1998) Russ Chem Bull 47:2379–2386
Shul’pin GB, Süss-Fink G, Lindsay Smith JR (1999) Tetrahedron 55:5345–5358
Shul’pin GB, Süss-Fink G, Shul’pina LS (2001) J Mol Catal A: Chem 170:17–34
Shul’pin GB, Nizova GV, Kozlov YN, Pechenkina IG (2002) New J Chem 26:1238–1245
Woitiski CB, Kozlov YN, Mandelli D, Nizova GV, Schuchardt U, Shul’pin GB (2004) J Mol Catal A: Chem 222:103–119
Shul’pin GB, Nizova GV, Kozlov YN, Arutyunov VS, dos Santos ACM, Ferreira ACT, Mandelli D (2005) J Organometal Chem 690:4498–4504
Mandelli D, Steffen RA, Shul’pin GB (2006) React Kinet Catal Lett 88:165–174
dos Santos VA, Shul’pina LS, Veghini D, Mandelli D, Shul’pin GB (2006) React Kinet Catal Lett 88:339–348
Nizova GV, Shul’pin GB (2007) Tetrahedron 63:7997–8001
Shul’pin GB, Matthes MG, Romakh VB, Barbosa MIF, Aoyagi JLT, Mandelli D (2008) Tetrahedron 64:2143–2152
Shul’pin GB, Kozlov YN, Kholuiskaya SN, Plieva MI (2009) J Mol Catal A: Chem 299:77–87
Lindsay Smith JR, Shul’pin GB (1998) Tetrahedron Lett 39:4909–4912
Nizova GV, Bolm C, Ceccarelli S, Pavan C, Shul’pin GB (2002) Adv Synth Catal 344:899–905
Süss-Fink G, Shul’pin GB, Shul’pina LS (2002) Process for the production of ketones. U.S. Patent 7,015,358, March 21, 2006 (Filed 2002, to Lonza A.-G., Switzerland). Eur Patent EP 1 385812 A0 (Application: WO 02/088063, art. 158 of the EPC)
Mandelli D, Woitiski CB, Schuchardt U, Shul’pin GB (2002) Chem Natur Comp 38:243–245
Kozlov YN, Mandelli D, Woitiski CB, Shul’pin GB (2004) Russ J Phys Chem 78:370–374
Romakh VB, Therrien B, Karmazin-Brelot L, Labat G, Stoeckli-Evans H, Shul’pin GB, Süss-Fink G (2006) Inorg Chim Acta 359:1619–1626
Romakh VB, Therrien B, Süss-Fink G, Shul’pin GB (2007) Inorg Chem 46:1315–1331
Shilov AE, Shul’pin GB (2000) Activation and catalytic reactions of saturated hydrocarbons in the presence of metal complexes. Dordrecht/Boston/London, Kluwer Academic Publishers
Shul’pin GB (2002) J Mol Catal A: Chem 189:39–66
Shul’pin GB (2003) Comptes Rendus, Chimie 6:163–178
Shul’pin GB (2004) Oxidations of C–H compounds catalyzed by metal complexes. In: Beller M, Bolm C (eds) Transition metals for organic synthesis, vol 2, Chap 2.2, 2nd edn, Wiley–VCH: Weinheim/New York, pp 215–242
Tanase S, Bouwman E (2006) Adv Inorg Chem 58:29–75
Sibbons KF, Shastri K, Watkinson M (2006) J Chem Soc Dalton Trans, 645–661
Shul’pin GB (2009) Mini-Rev Org Chem 6:95–104
Shul’pin GB (2001) Petrol Chem 41:405–412
Kozlov YN, Nizova GV, Shul’pin GB (2008) J Phys Org Chem 21:119–126
Mandelli D, Kozlov YN, Golfeto CC, Shul’pin GB (2007) Catal Lett 118:22–29
Veghini D, Bosch M, Fischer F, Falco C (2008) Catal Commun 10:347–350
Dorfman LM, Adams GE (1973) Reactivity of the hydroxyl radical in aqueous solutions, NSRDS-NBS 46, Washington DC
Farhataziz, Ross AB (1977) Selected specific rates of reactions of transients from water in aqueous solution. III. Hydroxyl radical and perhydroxyl radical and their radical ions. NSRDS-NBS 59, Washington DC
Shul’pin GB, Nizova GV, Kozlov YN, Gonzalez Cuervo L, Süss-Fink G (2004) Adv Synth Catal 346:317–332
Gómez L, Garcia-Bosch I, Company A, Sala X, Fontrodona X, Ribas X, Costas M (2007) Dalton Trans 5539–5545
Song WJ, Seo MS, George SD, Ohta T, Song R, Kang M-J, Tosha T, Kitagawa T, Solomon EI, Nam W (2007) J Am Chem Soc 129:1268–1277
Zhang R, Newcomb M (2008) Acc Chem Res 41:468–477
Serafimidou A, Stamatis A, Louloudi M (2008) Catal Commun 9:35–39
Balcells D, Raynaud C, Crabtree RH, Eisenstein O (2008) Inorg Chem 47:10090–10099
Ember E, Rothbart S, Puchta R, van Eldik R (2009) New J Chem 33:34–49
Castaman ST, Nakagaki S, Ribeiro RR, Ciuffi KJ, Drechsel SM (2009) J Mol Catal A: Chem 300:89–97
Bolm C, Meyer N, Raabe G, Weyhermüller T, Bothe E (2000) Chem Commun 2435–2436
Gilbert BC, Lindsay Smith JR, Mairata i Payeras A, Oakes J, Pons i Prats R (2004) J Mol Catal A: Chem 219:265–272
Lindsay Smith JR, Gilbert BC, Mairata i Payeras A, Murray J, Lowdon TR, Oakes J, Pons i Prats R, Walton PH (2006) J Mol Catal A: Chem 251:114–122
Sameera WMC, McGrady JE (2008) Dalton Trans 6141–6149
Kilic H, Adam W, Alsters PL (2009) J Org Chem 74:1135–1140
Stamatis A, Doutsi P, Vartzouma C, Christoforidis KC, Deligiannakis Y, Louloudi M (2009) J Mol Catal A: Chem 297:44–53
Lindsay Smith JR, Shul’pin GB (1998) Russ. Chem. Bull. 47:2313–2315
Kirillova MV, Kirillov AM, Mandelli D, Carvalho WA, Pombeiro AJL, Shul’pin GB (2010) J Catal 272:9–17
Acknowledgments
This work was supported by the Brazilian National Council on Scientific and Technological Development (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, CNPq, Brazil; grants Nos. 552774/2007-3, 478165/2006-4, 305014/2007-2), the State of São Paulo Research Foundation (Fundação de Amparo a Pesquisa do Estado de São Paulo, FAPESP; grant No. 2006/03996-6), and the Russian Foundation for Basic Research (grant No. 06-03-32344-a). L. S. S. and G. B. S. express their gratitude to the CNPq (grants No. 552774/2007-3 and 478165/2006-4), the FAPESP (grants Nos. 2006/03984-8, 2002/08495-4), and the Faculdade de Química, Pontifícia Universidade Católica de Campinas for making it possible for them to visit this University as invited scientists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shul’pin, G.B., Kozlov, Y.N., Shul’pina, L.S. et al. Oxidation of Reactive Alcohols with Hydrogen Peroxide Catalyzed by Manganese Complexes. Catal Lett 138, 193–204 (2010). https://doi.org/10.1007/s10562-010-0398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-010-0398-9