Abstract
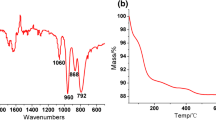
Pyridine(Py)-modified Keggin-type mono-vanadium-substituted heteropoly acids (Py n PMo11V, n = 1–4) were prepared by a precipitation method as organic/inorganic hybrid catalysts for direct hydroxylation of benzene to phenol in a pressured batch reactor and their structures were characterized by FT-IR. Among various catalysts, Py4PMo11V exhibited the highest catalytic activity (yield of phenol 9.0%) with the high selectivity for phenol, without observing the formation of catechol, hydroquinone and benzoquinone in the reaction with 80 vol% aqueous acetic acid, molecular oxygen and ascorbic acid used as the solvent, oxidant and reducing reagent, respectively. The influences of the reaction temperature, the pressure of oxygen, the amount of ascorbic acid, the amount of catalyst, and the reaction time on the yield of phenol were investigated to obtain the optimal reaction conditions for phenol formation. Pyridine can greatly promote the catalytic activity of the Py-free catalyst (H4PMo11VO40), mostly because the organic π electrons in the hydrid catalyst may extend their conjugation to the inorganic framework of heteropoly acid and thus dramatically modify the redox properties.






Similar content being viewed by others
References
Zheng ST, Yuan DQ, Zhang J, Yang GY (2007) Inorg Chem 46:4569
Zhao JW, Li B, Zheng ST, Yang GY (2007) Cryst Growth Des 7:2658
Liu YY, Murata K, Inaba M (2005) Catal Commun 6:679
Bardin BB, Davis RJ (1999) Appl Catal A Gen 185:283
Zhang FM, Yuan CS, Wang J, Zhu HY, Wang CY (2006) J Mol Catal A Chem 247:130
Zhang FM, Wang J, Yuan CS, Ren XQ (2005) Catal Lett 102:171
Yang YY, Xu L, Gao GG, Li FY, Qiu YF, Qu XS, Liu H (2007) Eur J Inorg Chem 2500
Zhang FQ, Zhang XM, Wu HS, Jiao HJ (2007) J Phys Chem A 111:159
San Felices L, Vitoria P, Gutierrez-Zorrilla JM, Lezama L, Reinoso S (2006) Inorg Chem 45:7748
Saha PK, Dutta B, Jana S, Bera R, Saha S, Okamoto K, Koner S (2007) Polyhedron 26:563
Bigi F, Corradini A, Quarantelli C, Sartori G (2007) J Catal 250:222
Neumann R, Khenkin AM (2006) Chem Commun 2529
San Felices L, Vitoria P, Gutierrez-Zorrilla JM, Reinoso S, Etxebarria J, Lezama L (2004) Chem Eur J 10:5138
Xu BB, Peng ZH, Wei YG, Powell DR (2003) Chem Commun 2562
Lu M, Wei YG, Xu BB, Cheung CFC, Peng ZH, Powell DR (2002) Angew Chem Int Ed 41:1566
Lemke K, Ehrich H, Lohse U, Berndt H, Jahnisch K (2003) Appl Catal A Gen 243:41
Niwa S, Eswaramoorthy M, Nair J, Raj A, Itoh N, Shoji H, Namba T, Mizukami F (2002) Science 295:105
Gu YY, Zhao XH, Zhang GR, Ding HM, Shan YK (2007) Appl Catal A Gen 328:150
Mishra GS, Kumar A (2002) Catal Lett 81:113
Laufer W, Niederer JPM, Hoelderich WF (2002) Adv Synth Catal 344:1084
Li Y, Xia HA, Fan FT, Feng ZC, van Santen RA, Hensen EJM, Li C (2008) Chem Commun 774
Zhong YK, Li GY, Zhu LF, Yan Y, Wu G, Hu CW (2007) J Mol Catal A Chem 272:169
Lee CH, Lin TS, Mou CY (2007) J Phys Chem C 111:3873
Kubacka A, Wang ZL, Sulikowski B, Corberan VC (2007) J Catal 250:184
Liu YY, Murata K, Inaba M (2006) J Mol Catal A Chem 256:247
Zhang FM, Guo MP, Ge HQ, Wang J (2007) Chin J Chem Eng 15:4
Ge HQ, Leng Y, Zhou CJ, Wang J (2008) Catal Lett. doi:10.1007/s10562-008-9464-y
Takahashi K, Okuhara T, Misono M (1985) Chem Lett 841
Misono M, Okuhara T, Ichiki T, Arai T, Kanda Y (1987) J Am Chem Soc 109:5535
Misono M (1987) Catal Rev Sci Eng 29:269
Wang J, Lin Z, Han SY, Eum M, Lee CW (2003) J Ind Eng Chem 9:281
Li W, Oshihara K, Ueda W (1999) Appl Catal A Gen 182:357
Song IK, Kaba MS, Barteau MA (1996) J Phys Chem 100:17528
Ishida MA, Masumoto Y, Hamada R, Nishiyama S, Tsuruya S, Masai M (1999) J Chem Soc Perkin Trans 2:847
Acknowledgments
The authors thank the Natural Science Foundation of China (Nos. 20306011 and 20476046) and the “Qinglan” Project of Jiangsu Province for Young Researchers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, H., Leng, Y., Zhang, F. et al. Direct Hydroxylation of Benzene to Phenol with Molecular Oxygen over Pyridine-modified Vanadium-substituted Heteropoly Acids. Catal Lett 124, 250–255 (2008). https://doi.org/10.1007/s10562-008-9506-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9506-5