Abstract
Given the possibility for disease transmission, this study was performed to determine whether there is detectable SARS-CoV-2 viral RNA in the blood of deceased tissue donors. A retrospective analysis of blood samples from eligible deceased tissue donors from Oct 2019 through June 2020 was performed. Plasma aliquots were initially tested with a SARS-CoV-2 NAT Assay; positive samples were further tested using an alternate NAT and an antibody assay. The proportion of donors with confirmed RNAemia and 95% confidence intervals were computed. Of donor samples collected in 2019, 894 yielded valid results, with 6 initially positive, none of which confirmed positive by alternate NAT. Of donor samples collected in 2020, 2562 yielded valid initial NAT results, with 21 (0.8%) initially positive. Among those, 3 were confirmed by alternate NAT, 17 were not confirmed, and 1 had an invalid alternate NAT result. The rate of SARS-CoV-2 RNAemia in deceased tissue donors is approximately 1 per 1000, and it is unknown whether this RNAemia reflects the presence of infectious virus. Given these results, the risk of transmission through tissue is thought likely to be low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the COVID-19 pandemic was declared in March 2020, much has been learned, and much remains to discover about COVID-19 and its cause, SARS-CoV-2. As of July 3, 2022, more than 549 million people worldwide are confirmed to have been infected, and more than 6 million have perished (Johns Hopkins University Medicine 2022). In the United States, the death toll from COVID-19 has surpassed that of the 1918 Flu Pandemic, killing over 1 million Americans, with numbers increasing daily. Two years after SARS-CoV-2 was first identified, transmission has not subsided, variants with increased transmissibility have emerged, and the virus appears to be detectable beyond the respiratory tract. Further, damage from COVID-19 has been observed in a multitude of tissues and organs such as the brain, olfactory nerves, myocardium, kidneys, arteries, and intestines, although live viral effect has not been proven (Xu et al. 2021).
It is imperative to understand the possible risks of transmission via tissue and organ transplantation. After reports indicated that SARS-CoV-2 was detectable in blood by nucleic acid amplification testing (NAT) in mildly ill or asymptomatic individuals, including in asymptomatic blood donors (Huang et al. 2020; Bakkour et al. 2021), there was concern that viremia (if present) could result in transmission through transfusion and transplantation, because many transfusion-transmitted infections (TTIs) are also transmitted via transplantation. While in vitro infectivity studies have not yet been performed using NAT positive blood samples, the presence of RNA in the blood (i.e., RNAemia) raises concern of potential transmission through tissue and organ transplantation. Since that time, autopsy and other studies have detected viral RNA and visualized intact viral particles in multiple organs, including kidneys, the gastrointestinal tract, male genitourinary tract, and amniotic membrane (Bradley et al. 2020; Best et al. 2021; Gaussen et al. 2021; Penfield et al. 2020; Trypsteen et al. 2020).
Tissue donors are evaluated for communicable disease risks to minimize potential transmission associated with tissue transplantation. It is not known at this time how viable SARS-CoV-2 is when located outside the respiratory tract, the frequency or length of viremia, and how frequently the virus is found in, or whether transmissible by, tissues outside the respiratory tract. Given the challenges with identifying viral infection based solely on symptoms and the transmission of this virus from asymptomatic individuals, we conducted this study to determine whether there is detectable viral RNA in the blood of deceased tissue donors. We sought to gauge the risk of SARS-CoV-2 transmission through tissue by detecting blood RNAemia of eligible deceased tissue donors. American Association of Tissue Banks’ (AATB) recommendations in effect during most of the study period indicated that in order to donate, donors should be free of symptoms of SARS-CoV-2 infection and not have had close contact with a person who had confirmed infection, in addition to the traditional screening for health and social behaviors that are exclusionary (American Association of Tissue Banks 2020). This study evaluating the presence of RNA-emia in deceased tissue donors (“donor/s”) is a first step in developing data regarding the risk of SARS-CoV-2 viremia in accepted tissue donors, needed to inform prudent policy on donation.
Materials and methods
Participating tissue banks with donors from multiple areas of the US and Canada provided a de-identified list of all available deceased donor specimens collected and retained frozen during the study period (October 1, 2019, to June 30, 2020). Because specimens were de-identified, it is not possible to know how many donors were both organ and tissue donors, whether specimens were collected before or after death (the study assumes worst-case-scenario that specimens were collected after death), and no results were provided to participating tissue banks. Specimens were considered for inclusion according to study criteria (Table 1), and then selected for testing as follows. For specimens collected during 2019 (control arm, tested to estimate the baseline assay specificity during a time when the pre-test probability of SARS-CoV-2 was anticipated to be zero), all specimens were included from four tissue banks that had fewer than 100 specimens available, while a random selection was made from available specimens of the remaining tissue banks to target a total sample size of 1000 for 2019. The testing algorithm (Fig. 1) in this study relied on a screening NAT assay, confirmation of initial positive samples by an alternate NAT assay (performed by Grifols, San Diego, CA) to determine final NAT disposition, and when adequate sample was available, also testing initial positives for anti-SARS-CoV-2 antibodies. Initial NAT and antibody testing were performed by Creative Testing Solutions (CTS) (Tempe, AZ).
Testing Algorithm Overview. Initial NAT was performed first. If initial NAT yielded no test results, the specimen result is reported as “No result” and no further testing was performed. If the initial NAT test was nonreactive, the specimen result is reported as “NAT Negative” and no further testing was performed. Initial NAT-reactive specimens were further tested with an alternate NAT to determine the final NAT disposition. NAT results are reported as “NAT Unconfirmed” if there was not an alternate NAT result, “NAT Negative” if the alternate NAT was non-reactive,” and “NAT Positive” if the alternate NAT result was reactive. Initial NAT-reactive specimens were also tested for antibodies to SARS-CoV-2. Antibody results were reported as “No Ab result” if there was insufficient volume to perform testing or a test failure, “Ab positive” if the assay was reactive, and “Ab negative” if the assay was nonreactive. Initial NAT and antibody testing performed at Creative Testing Solutions (CTS), and alternate NAT performed at Grifols
After excluding ineligible samples sent to the testing laboratory in error, 930 2019 samples were tested, and 894 had valid test results and were included in the analysis. All specimens collected in 2020 meeting eligibility criteria were tested (2660), and all 2561 specimens that yielded valid test results were included in the analysis. Samples without test results either had insufficient volume, a test failure occurred, or was erroneously not tested (Fig. 2).
Archive donor specimens in aliquot tubes accessed for this study had been stored at − 30 to − 35 °C. Donor specimens were shipped to CTS on dry ice, and remained frozen at ≤ 20 °C until it was time for specimens to be aliquoted for testing. Specimens were prepared for testing by thawing them, aliquoting them into appropriate low volume testing tubes labeled with unlinked sample ID, and refrozen at ≤ 20 °C until being shipped on dry ice to another CTS site that performed testing. Samples were then thawed (second freeze/thaw cycle), centrifuged, and then routed for testing.
Aliquots labeled as plasma (originally collected in EDTA) were tested with the Grifols Research Use Only (RUO) Procleix SARS-CoV-2 Assay on the Procleix Panther System (“screening NAT”) (Bakkour et al. 2021; Sauleda et al. 2022; Grifols 2022). The Procleix SARS-CoV-2 Assay is a qualitative in vitro nucleic acid test that uses transcription mediated amplification (TMA) for the detection of SARS-CoV-2 nucleic acid. The assay is a highly sensitive and specific test for the detection of SARS-CoV-2 RNA on a high-throughput, automated platform. An evaluation of the performance characteristics can be found in Sauleda et al. (2022). Results obtained from negative and positive calibrators are used to determine the validity of the run and to establish the assay cutoff values for the internal control signal and the analyte signal. Reactive or nonreactive results for each specimen tested are provided by the instrument based on predetermined calculations of the cutoff values that are coded within the assay software. A single test was performed on each sample and results were evaluated for reactivity. Aliquots from donors with reactive initial NAT results were sent to Grifols for “alternate NAT” testing using Grifols Research Use Only (RUO) SARS-CoV-2 NAT, a lab-developed supplemental TMA assay that targets a different region of the SARS-CoV-2 genome, to verify reactivity (Sauleda et al. 2022). The “alternate NAT” assay is a qualitative in vitro nucleic acid test with demonstrated comparable sensitivity to the “screening NAT” (Sauleda et al. 2022). For some initial reactive donations, serum aliquots were used for the alternate assay due to insufficient residual plasma volume. A single sample was not routed for alternate NAT testing due to insufficient volume. Reactive samples (plasma or serum aliquots), if volume available, were also routed for antibody testing using the Ortho Clinical Diagnostics EUA VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total test. The Ortho Clinical Diagnostics VITROS® platform uses immunometric technology for qualitative measurement of total antibody (IgG and IgM) to SARS-CoV-2. All results were submitted to Vitalant Research Institute (VRI) for further evaluation.
Specimen collection date, the donor’s age in years at death, sex, race/ethnicity, zip code of residence, and state/province were collected in deidentified form, i.e., linked only to the tested specimens. The proportion of donors with confirmed blood RNAemia was computed and Wilson score 95% confidence intervals are reported.
Results
The study population is not representative of the demographics of the general population of the United States or Canada, with the study population heavily skewed towards male (68.2%) and white (69.6%) tissue donors. Hispanic ethnicity could be ascertained for a small minority of donors in the study. Although all Census regions of the United States and the Canadian province of Nova Scotia are represented, a large majority of donors resided in the South (64.8%) (Table 2).
As represented in Fig. 2, a total of 3455 unique donor samples provided evaluable, valid data, i.e., a non-reactive initial NAT result or a reactive initial NAT result and a valid alternate NAT result; 134 additional samples did not yield valid screening NAT results (Table 3), and one was initially reactive but unconfirmed because alternate NAT did not yield a valid result. This sample was kept in the descriptions of the study population, but was not in the computation of proportions reactive. Of the 3455 donor samples with valid initial and alternate NAT results, 894 were collected in the last quarter of 2019 and 2561 during the first half of 2020.
Twenty-seven samples that were reactive on the initial NAT were routed to Grifols for alternate NAT testing. For 7 of these, serum aliquots were used due to insufficient residual plasma volume for the alternate assay, and one sample had insufficient volume for alternate NAT testing.
Of the 894 unique donor blood samples collected in 2019 yielding valid initial NAT results, 6 were initially reactive, and 888 non-reactive. None or 0.00% were confirmed reactive for SARS-CoV-2 RNA (95% confidence interval [CI] 0.00–0.43%); one sample did not confirm reactive but did have a positive Ab result. Of the 2562 unique donor samples collected in 2020 yielding valid initial NAT results, 21 were initially reactive, and 2541 non-reactive. In alternate NAT testing, three or 0.12% (95% CI: 0.04–0.34%) were confirmed RNAemic, 1 was yielded an invalid alternate NAT result (therefore disposition of “NAT unconfirmed”), and the remainder were nonreactive.
Among all donor samples tested by initial NAT (including both 2019 and 2020), 125 samples eligible for inclusion had insufficient volume for initial NAT testing, 5 yielded an invalid initial NAT test result (test failure), and four samples were erroneously not tested using initial NAT. A further sample from 2020 tested initially reactive, but alternate NAT testing yielded an invalid result. Because of small sample size, donor demographics for positive samples are not being reported.
Of all 27 initially NAT reactive samples, 17 had sufficient volume available for antibody testing using the Ortho assay. One confirmed NAT positive sample (2020), and one initially reactive sample that was not confirmed positive by alternate NAT (2019), were positive for anti-SARS-CoV-2 Spike antibodies.
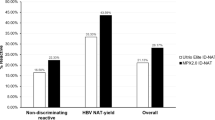
Figure 3 shows the distribution of signal-to-cutoff ratios (S/COs) obtained using the screening NAT assay, and final status based on alternate NAT and serology. The confirmed NAT positive samples all had high S/COs in initial NAT testing relative to the S/COs of initially reactive samples that were not confirmed. The figure includes the single sample that did not have a valid alternate NAT result.
S/CO values for all Initial NAT results compared to final test results. A represents 2019 specimen results, while B represents 2020 specimen results. The y axis represents the Signal/Cut Off (S/CO) ratio values obtained on the initial NAT assay. The x axis organizes S/CO values among initial NAT reactive (R) vs initial NAT non-reactive (NR), while also indicating (by color, as labeled) both the final NAT disposition and the Antibody results. See Fig. 1 for results description. The S/CO cutoff between positive and negative results is 1.0. Positive results close to the assay cutoff generally have a higher likelihood of being false-positive than those with higher S/CO values
Discussion
This is the first known study publishing data regarding the presence of SARS CoV-2 RNA in the blood of deceased tissue donors. Between January 1 and June 30, 2020, among the included tissue donors (who were not suspected at the time of having COVID-19 and no vaccine was available), there were 3 confirmed positive donor samples by alternate NAT testing among 26 initial positives that had a confirmatory test result, about 0.12% of donors tested. Given the relatively high S/CO values of the initial positive results, that then also confirmed positive on the alternate NAT, and high specificity of the confirmed assays, our data support the strong likelihood that the 3 positive confirmed samples represent RNAemia. However, this is a low confirmation rate and possible causes for lack of confirmation include interfering substances in deceased donor plasma, issues related to sample quality after storage, differences in assay sensitivity, or other/unresolved assay issues. It should be noted that we observed a higher initial NAT reactive proportion in specimens collected during 2020, of 1.2% compared to 0.7% in 2019 when SARS-CoV-2 was likely not circulating in the population. This suggests that a small number of the initially reactive samples collected in 2020 that failed to confirm on the alternate NAT assay may have been true positives, including the 2019 specimen that did not confirm positive but had positive antibody results.
The study data suggest an overall rate of RNAemia of about 1 per 1000 in the US deceased donor population during January through June 2020, and possible risk of viremia in donors from which tissues are recovered. Additionally, among the 17 initial NAT positive samples that were tested by serology, two were positive for anti-SARS-CoV-2 antibodies [one from 2019 NAT(−)/Ab(+) and one from 2020 NAT(+)/Ab(+)], suggesting that the remaining two of the confirmed NAT(+) donors likely had infections early in course of disease, before an antibody response could develop.
In comparison with blood donors tested using residual donor plasma minipools (MPs), we found a higher rate of RNAemia in deceased tissue donors, by a log factor of about 2 (100,000 vs 1000) (Bakkour et al. 2021). Testing with MPs may have resulted in lower detection amongst blood donors, particularly if any of the samples tested had low RNA concentrations (below ~ 100–300 copies/mL) (Bakkour et al. 2021). Blood donor communicable disease screening risk assessments are performed via first-person donor screening interviews and are considered more likely accurate compared to deceased donor screening risk assessments performed by obtaining information from others (e.g., next-of-kin). Furthermore, deceased donors sometimes have a poorly characterized cause of death; autopsies are not required. Estimates of RNAemia determined by blood donors reporting post donation information (PDI) indicative of respiratory illness (subsequent to the donation at a time when RNAemia is more likely to represent true viremia with replicating virus) during the pandemic suggests a higher rate of COVID-19 RNA detection compared with either the blood donor population or deceased tissue donors13 (Cappy et al. 2020).
Since the presence of RNA (RNAemia) does not imply transmissible live virus, the rate of true viremia among tissue donors is unknown but may be less than 1 in 1000. This is based on lack of infectious virus in COVID-19 patients with detected RNA in blood samples (Andersson et al. 2020). There were no samples available in our study for infectivity testing from the 3 confirmed samples. For two samples, quantity was not sufficient, and for the third, the sample was lost. The infectivity of blood and tissues where SARS CoV-2 RNA is detected is an area of active research and would be very helpful in considering tissue and organ transplantation risks.
Despite the potential risk that would be associated with viremia (if present), no transmission has been detected through blood or tissue products (Bakkour et al. 2021; Gaussen et al. 2021). Based on the current data, it is unlikely that SARS-CoV-2 is readily transmitted through tissue products, especially those that undergo processing. Studies have been performed for other respiratory viruses in blood donors, including influenza, and have not found evidence of transfusion transmission despite RNAemia (Likos et al. 2007; Dos Santos et al. 2020). Further studies examining the presence of RNA in unprocessed tissue recovered from SARS-CoV-2 nasal swab-positive donors are underway to provide tissue tropism information.
For solid organs, only lung transplantation has been proven to transmit SARS-CoV-2, with a possible, but unlikely, transmission via liver transplantation (Gaussen et al. 2021; Kaul et al. 2021; Heinz et al. 2020). Because donors in this study were anonymized, it is unknown how many of the study donors were also organ donors. Given the partial overlap between organ and tissue donors, the presence of RNAemia in organ donors should be considered possible. Upper respiratory samples were tested on all organ donors beginning around April 2020, and at the time no organ donors were accepted that had positive results for SARS CoV-2. While the significance of potential RNAemia is unknown, given there were 12,588 deceased organ donors in 2020 (Organ Procurement and Transplantation Network 2022) with such few transmissions, the data are reassuring. Now that some non-lung organ transplantation is being performed on SARS-CoV-2 positive organ donors, as part of the outcomes data collection there may be an opportunity to further investigate whether RNAemia or viremia is present at the time of transplantation (OPTN 2022).
There are important limitations to this study. The study necessarily used investigational assays under Emergency Use Authorization (EUA), and while the performance characteristics were not known, a recent peer-reviewed study has been published11. Performance characteristics of the assays on our study sample matrix, which were all collected after cessation of the heartbeat (from deceased donors, e.g.., “cadaveric” or “post-mortem” specimens), are still unknown. Furthermore, there were 134 samples that did not yield valid NAT screening results. The low rate (0.7%) of initial positive, unconfirmed results in 2019, when no virus was known to be circulating in the US, is reassuring concerning the specificity of the screening assay. Deceased donor specimen quality tends to be poor compared to those obtained from living or heart-beating individuals, and such factors as hemolysis, inhibitors, proteolysis, and hemodilution can affect assay results (Greenwald et al. 2018). The results may not be representative of the tissue donor population in the U.S., as results were obtained from a convenience sample of tissue banks who volunteered to participate. These results only apply to deceased tissue donors, given that samples tested were from deceased donor specimens—organ-only donors and living donors were not evaluated in this study. Furthermore, only donors that were not suspected of having COVID-19 were eligible for study participation, and rejected donors may have had a higher rate of RNAemia.
This study was made possible because of the willingness of tissue banks to participate, and due to the availability of study material because of voluntary policies regarding donor specimen storage. Funding was provided by the American Association of Tissue Banks (AATB), an industry trade organization, and in-kind contributions. There are no requirements by either AATB or the Food and Drug Administration (FDA) for storage of donor specimens. Furthermore, there are also no repositories of specimens from either tissue donors or recipients that are supported for research, unlike that which exists for blood donors, including National Heart, Lung, and Blood Institute’s Recipient Epidemiology and Donor Evaluation Study Program (REDS) (Kleinman et al. 2014), Centers for Disease Control and Prevention’s National Blood Donor Seroprevalence Study (NBDS) (Jones et al. 2021), and FDA’s Transfusion-Transmissible Infections Monitoring System (TTIMS) (Custer et al. 2016). We strongly encourage further research to include systematically evaluating the presence of SARS CoV-2 (and future emerging infectious diseases) in various tissues and organs, transmissibility studies of blood with SARS CoV-2 RNAemia, and provision of National Institutes of Health (NIH)-sponsored prospective specimen collection and storage from organ and tissue donors for use in research to inform donor evaluation in the face of future emerging infectious diseases. Such studies should be routinely considered early in the evaluation of emerging infectious diseases, as is currently done with blood donation.
In conclusion, SARS-CoV-2 RNA can be present in plasma and serum samples from deceased tissue donors, although incidence is low. The potential for transmission will be further elucidated with focused study on persistence of the virus in stored human tissue.
Abbreviations
- AATB:
-
American Association of Tissue Banks
- CI:
-
Confidence interval
- EUA:
-
Emergency use authorization
- MP:
-
Minipools
- NAT:
-
Nucleic acid amplification test
- NBDS:
-
National Blood Donor Seroprevalence Study
- NIH:
-
National Institutes of Health
- REDS:
-
Recipient Epidemiology and Donor Evaluation Study Program
- RUO:
-
Research use only
- TMA:
-
Transcription-mediated nucleic acid amplification
- TTIMS:
-
Transfusion-Transmissible Infections Monitoring System
- VRI:
-
Vitalant Research Institute
References
Andersson MI, Arancibia-Carcamo CV, Auckland K, et al. (2020) SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res 5:181. https://doi.org/10.12688/wellcomeopenres.16002.2. Published 2020 Oct 12
American Association of Tissue Banks (2020) AATB Bulletin 20-3. Published January 31, 2020. Online. https://www.aatb.org/bulletin-20-3
Bakkour S, Saá P, Groves JA et al (2021) Minipool testing for SARS-CoV-2 RNA in United States blood donors. Transfusion 61(8):2384–2391. https://doi.org/10.1111/trf.16511
Best JC, Kuchakulla M, Khodamoradi K, Lima TFN, Frech FS, Achua J, Rosete O, Mora B, Arora H, Ibrahim E, Ramasamy R (2021) Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health 39(3):489–495. https://doi.org/10.5534/wjmh.200192. Epub 2021 Feb 24. PMID: 33663031; PMCID: PMC8255403
Bradley BT, Maiolo H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Marshall D, Lacy JM, Williams T, Yarid N (2020) Histopathology and ultrastructural findings of fatal COVID-19 infections. Lancet 396(10247):320–332. https://doi.org/10.1016/S0140-6736(20)31305-2
Cappy P, Candotti D, Sauvage V et al (2020) No evidence of SARS-CoV-2 transfusion transmission despite RNA detection in blood donors showing symptoms after donation. Blood 136(16):1888–1891. https://doi.org/10.1182/blood.2020008230
Custer B, Stramer SL, Glynn S, Williams AE, Anderson SA (2016) Transfusion-transmissible infection monitoring system: a tool to monitor changes in blood safety. Transfusion 56(6 Pt 2):1499–1502. https://doi.org/10.1111/trf.13632 (PMID: 27295025)
Dos Santos Bezerra R, de Melo Jorge DM, Castro ÍA, Moretto EL, Scalon de Oliveira L, Ubiali EMA, Covas DT, Arruda E, Kashima S, Slavov SN (2020v) Detection of influenza A(H3N2) virus RNA in donated blood. Emerg Infect Dis 26(7):1621–1623. https://doi.org/10.3201/eid2607.200549. Epub 2020 Jun 21. PMID: 32304372; PMCID: PMC7323509
Gaussen A, Hornby L, Rockl G et al (2021) Evidence of SARS-CoV-2 infection in cells, tissues, and organs and the risk of transmission through transplantation. Transplantation 105(7):1405–1422. https://doi.org/10.1097/TP.0000000000003744
Greenwald MA, Kerby S, Francis K, Noller AC, Gormley WT, Biswas R, Forshee RA (2018) Detection of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus in postmortem blood specimens using infectious disease assays licensed for cadaveric donor screening. Transpl Infect Diseases. 20(1):e12725
Grifols (2022) Procleix SARS-CoV-2 Assay. U.S. Food and Drug Administration website. https://www.fda.gov/media/145938/download. Revision GDSS-IFU-000049-REG v. 4.0. Accessed 4 May 2022
Heinz N, Griesemer A, Kinney J, Vittorio J, Lagana SM, Goldner D, Velasco M, Kato T, Lobritto S, Martinez M (2020) A case of an Infant with SARS-CoV-2 hepatitis early after liver transplantation. Pediatr Transpl 24(8):e13778. https://doi.org/10.1111/petr.13778. Epub 2020 Jun 25. PMID: 32559354; PMCID: PMC7323125
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, Di Germanio C, Green V, Notari E, Saa P, Biggerstaff BJ, Strauss D, Kessler D, Vassallo R, Reik R, Rossmann S, Destree M, Nguyen KA, Sayers M, Lough C, Bougie DW, Ritter M, Latoni G, Weales B, Sime S, Gorlin J, Brown NE, Gould CV, Berney K, Benoit TJ, Miller MJ, Freeman D, Kartik D, Fry AM, Azziz-Baumgartner E, Hall AJ, MacNeil A, Gundlapalli AV, Basavaraju SV, Gerber SI, Patton ME, Custer B, Williamson P, Simmons G, Thornburg NJ, Kleinman S, Stramer SL, Opsomer J, Busch MP (2021) Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA 326(14):1400–1409. https://doi.org/10.1001/jama.2021.15161 (PMID: 34473201; PMCID: PMC8414359)
Johns Hopkins University & Medicine (2022) Coronavirus Resource Center. https://coronavirus.jhu.edu/. Accessed 07 Mar 2022
Kaul DR, Valesano AL, Petrie JG et al (2021) Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transpl 21(8):2885–2889. https://doi.org/10.1111/ajt.16532
Kleinman S, Busch MP, Murphy EL, Shan H, Ness P, Glynn SA, National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III) (2014) The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion 54(3 Pt 2):942–955. https://doi.org/10.1111/trf.12468. Epub 2013 Nov 4. PMID: 24188564; PMCID: PMC4383641
Likos AM, Kelvin DJ, Cameron CM, Rowe T, Kuehnert MJ, Norris PJ, National Heart, Lung, Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II) (2007) Influenza viremia and the potential for blood-borne transmission. Transfusion 47(6):1080–1088. https://doi.org/10.1111/j.1537-2995.2007.01264.x. PMID: 17524100
Organ Procurement and Transplantation Network (OPTN) National Data. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed 13 Feb 2022.
OPTN (2022) Summary of Current Evidence and Information—Donor SARS-CoV-2 Testing and Organ Recovery from Donors with a History of COVID-19. Version Release Date: January 21, 2022. Online. https://optn.transplant.hrsa.gov/media/kkhnlwah/sars-cov-2-summary-of-evidence.pdf. Accessed 13 Feb 2022
Penfield CA, Brubaker SG, Limaye MA, Lighter J, Ratner AJ, Thomas KM, Meyer JA, Roman AS (2020) Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM 2(3):100133. https://doi.org/10.1016/j.ajogmf.2020.100133. Epub 2020 May 8. PMID: 32391518; PMCID: PMC7205635
Sauleda S, Palacios L, Brès V, Piñana M, Alonso-Hernandez L, Bes M, Piron M, Entrena E, Minguez-Micolau AM, Marimón JM, Gurrola A, Soria G, Puig L, Antón A, Pumarola T, Linnen JM (2022) Clinical evaluation of the Procleix SARS-CoV-2 assay, a sensitive, high-throughput test that runs on an automated system. Diagn Microbiol Infect Dis 102(1):115560. https://doi.org/10.1016/j.diagmicrobio.2021.115560. Epub 2021 Sep 25. PMID: 34688036; PMCID: PMC8464401
Trypsteen W, Van Cleemput J, Wv S, Gerlo S, Vandekerckhove L (2020) On the whereabouts of SARS-CoV-2 in the human body: a systematic review. PLoS Pathog 16(10):e1009037. https://doi.org/10.1371/journal.ppat.1009037
Xu D, Zhou F, Sun W, Chen L, Lan L, Li H, Xiao F, Li Y, Kolachalama VB, Li Y, Wang X, Xu H (2021) Relationship between serum severe acute respiratory syndrome coronavirus 2 nucleic acid and organ damage in coronavirus 2019 patients: a cohort study. Clin Infect Dis 73(1):68–75. https://doi.org/10.1093/cid/ciaa1085.PMID:32720678;PMCID:PMC7454386
Acknowledgements
This study was funded by the American Association of Tissue Banks, with a special thank you to Beverly Bliss for your support and expert management. This study could not have happened without the participating tissue establishments: Cryolife, HemaQuebec, LifeNet Health, Lifelink Foundation, MTF Biologics, Pinnacle Transplant Technologies, The Regional Tissue Bank (Nova Scotia), RTI Surgical, UT Southwestern, Vivex.
Funding
This study was funded by the American Association of Tissue Banks.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Valerie Green and Eduard Grebe. The first draft of the manuscript was written by Melissa Greenwald and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Greenwald, M.A., Grebe, E., Green, V. et al. Low rate of detection of SARS-CoV-2 RNA in deceased tissue donors. Cell Tissue Bank 24, 585–596 (2023). https://doi.org/10.1007/s10561-022-10054-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-022-10054-0