Abstract
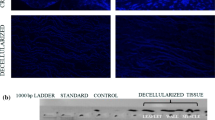
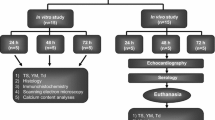
Cryopreserved pulmonary homograft (CPH) implantation remains the gold standard for reconstruction of the right ventricular outflow tract (RVOT). Harvesting homografts < 24-h post mortem is the international norm, thereby largely excluding cadaveric donors. This study examines the structural integrity and stability of ovine pulmonary homografts harvested after a 48-h post mortem period, cryopreserved and then implanted for up to 180 days. Fifteen ovine pulmonary homografts were harvested 48-h post mortem and cryopreserved. Five CPH served as a control group (group 1; n = 5). CPH were implanted in the RVOT of juvenile sheep and explanted after 14 days (group 2; n = 5) and 180 days (group 3; n = 5). Leaflet integrity was evaluated by strength analysis, using tensile strength (TS), Young’s modulus (YM) and thermal denaturation temperature (Td), and morphology, including haematoxylin and eosin (H&E), Picrosirius red staining, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and von Kossa stains. Echocardiography confirmed normal function in all implants. In explants, no reduction in TS, YM or Td could be demonstrated and H&E showed mostly acellular leaflet tissue with no difference on Picrosirius red. TEM demonstrated consistent collagen disruption after cryopreservation in all three groups, with no morphological deterioration during the study period. von Kossa stains showed mild calcification in group 3. No deterioration of structural integrity could be demonstrated using strength or morphological evaluations between the controls and implant groups over the study period. Extending the post mortem harvesting time of homografts beyond 24 h did not appear to negatively affect the long-term performance of such transplanted valves in this study.





Similar content being viewed by others
References
Angell WW, Oury JH, Lamberti JJ, Koziol J (1989) Durability of the viable aortic homograft. J Thorac Cardiovasc Surg 98:48–56
Axelsson I, Malm T (2018) Long-term outcome of homograft implants related to donor and tissue characteristics. Ann Thorac Surg 106(1):165–171
Barrett-Boyes B (1987) 25 years clinical experience of homograft surgery—a time for reflection 1962–1987. In: Yankah AC, Hetzer R, Miller DC, Ross DN, Somerville J, Yacoub MH (eds) Cardiac valve allografts. Springer, New York, pp 347–358
Botes L, van den Heever JJ, Smit FE, Neethling WML (2012) Cardiac homografts: a 24-year South African experience. Cell Tissue Bank 13:139–146
Brubaker S, Lotherington K, Zhao J, Hamilton B, Rockl G, Duong A, Garibaldi A, Simunovic N, Alsop D, Dao D, Bessemer R, Ayeni OR (2016) Tissue recovery practices and bioburden: a systematic review. Cell Tissue Bank 17:561–571
Dagum P, Green GR, Timek TA, Daughters GT, Foppiano LE, Tye TL, Bolger AF, Ingels NB, Miller DG (1999) Functional evaluation of the Medtronic stentless porcine xenograft mitral valve in sheep. Circulation 100(19 Suppl. 2):70–77
Dawson PE, Brockbank KGM (1997) Human heart cold ischemia and its effects on post cryopreservation viability of heart valves. In: Yankah AC, Yacoub MH, Hetzer R (eds) Cardiac valve allografts, science and practice. Springer, New York
Green MK, Walsh MD, Dare A, Hogan PG, Zhao XM, Frazer IH, Bansai AS, O’Brien MF (1998) Histologic and immunohistochemical responses after aortic valve allografts in the rat. Ann Thorac Surg 66:S216–S220
Hechadi J, Gerber BL, Coche E, Melchior J, Jashari R, Glineur D, Noirhomme P, Rubay J, El Khoury G, De Kerchove L (2013) Stentless xenografts as an alternative to pulmonary homografts in the Ross operation. Eur J Cardiothorac Surg 44:e32–e39
Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals [online]. http://www.nap.edu/catalog/5140.html. Accessed 20 Jan 2016
Langley SM, Livesey SA, Tsang VT, Barron DJ, Lamb RK, Ross JK, Monro JL (1996) Long-term results of valve replacement using antibiotic-sterilised homografts in the aortic position. Eur J Cardiothorac Surg 10(12):1097–1106
Methe H, Hess S, Edelmann ER (2007) Endothelial immunogenicity—a matter of matrix microarchitecture. Thromb Haemost 98:278–282
Mitchell RN, Jonas RA, Schoen FJ (1998) Pathology of explanted cryopreserved allograft heart valves: comparison with aortic valves from orthotopic heart transplants. J Thorac Cardiovasc Surg 115:118–127
Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC (2008) Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg 16(10):559–565
O’Brien MF, Stafford G, Gardner M, Pohlner P, McGiffin D, Johnston N, Brosnan A, Duffy P (1987a) The viable cryopreserved allograft aortic valve. J Cardiac Surg 2(Suppl 1):153–167
O’Brien MF, Stafford EG, Gardner MAH, Pohlner P, McGiffin D (1987b) A comparison of aortic valve replacement with viable cryopreserved and fresh allograft valves, with a note on chromosomal studies. J Thorac Cardiovasc Surg 94:812–823
O’Brien MF, Stafford GE, Gardner MAH (1995) Allograft aortic valve replacement: long-term follow-up. Ann Thorac Surg 60:S65–S70
O’Brien MF, Harrocks S, Stafford EG, Gardner MAH, Pohlner PG, Tesar PJ, Stephens F (2001) The homograft aortic valve: a 29-year, 99.3% follow up of 1022 valve replacements. J Heart Valve Dis 10(3):334–344
Rüegg M, Moor U, Blanc B (1975) Hydration and thermal denaturation of beta-lactoglobulin. A calorimetric study. Biochim Biophys Acta 400:334–342
Schenke-Layland K, Madershahian N, Riemann I, Starcher B, Halbhuber KJ, König K, Stock UA (2006) Impact of Cryopreservation on extracellular matrix structures of heart valve leaflets. Ann Thorac Surg 81:918–926
Smit F (2011) The effect of pre-harvest ischemic time on valvular homografty performance - an experimental study. PhD. University of the Free State
Smit FE, Bester D, van den Heever JJ, Schlegel F, Botes L, Dohmen PM (2015) Does prolonged post-mortem cold ischemic harvesting time influence cryopreserved pulmonary homograft tissue integrity? Cell Tissue Bank 16(4):531–544
Smith SH, Judge MD (1991) Relationship between pyridinoline concentration and thermal stability of bovine intramuscular collagen. J Anim Sci 69:1989–1993
Spurr AR (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Thubrikar MJ, Deck JD, Aouad J, Nolan SP (1983) Role of mechanical stress in calcification of aortic bio prosthetic valves. J Thorac Cardiovasc Surg 86:115–125
Yacoub M, Kittle CF (1970) Sterilization of valve homografts by antibiotic solutions. Circulation 41:SII29–SII31
Yacoub M, Rasmi NRH, Sundt TM, Lund O, Boyland E, Radley-Smith R, Khaghani A, Mitchell A (1995) Fourteen-year experience with homovital homografts for aortic valve replacement. J Thorac Cardiovasc Surg 110:186–194
Yankah AC, Hetzer R (1987) Procurement and viability of cardiac homografts. In: Yankah AC, Hetzer R, Miller C, Ross DN, Somerville J, Yacoub MH (eds) Cardiac valve allografts 1962–1987. Springer, New York, pp 23–26
Yoshikawa Y, Kitamura S, Taniguchi S, Kameda Y, Niwaya K, Sakaguchi H (2000) Pulmonary ventricular outflow reconstruction with a size-reduced cryopreserved pulmonary valve allograft: mid-term follow-up. Jpn Circ J 64:23–26
Zouhair S, Aguiari P, Iop L, Korossis S, Wolkers WF, Gerosa G (2017) Advanced preservation methodologies for decellularized cardiovascular scaffolds. In: 26th Congress of the European association of tissue banks, Treviso, Italy. https://www.eatb.org/images/2017/Final-Program_EATB2017_web.pdf. Accessed Sept 2018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bester, D., Botes, L., van den Heever, J.J. et al. Cadaver donation: structural integrity of pulmonary homografts harvested 48 h post mortem in the juvenile ovine model. Cell Tissue Bank 19, 743–754 (2018). https://doi.org/10.1007/s10561-018-9729-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-018-9729-7