Abstract
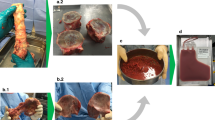
NHS Blood and Transplant Tissue and Eye Services (TES) and Scottish National Blood Transfusion Services Tissues and Cells Directorate (TCD) currently bank whole, frozen femoral head bone from living donors who are undergoing primary hip replacement surgery. When required, the bone is issued to a surgeon still frozen on dry ice (− 79 °C). Consequently, the femoral head bone is not processed, is not sterilised and at the time of issue, it contains donor blood, bone marrow and associated cells. We have previously shown that, cut, shaped and washed bone from deceased donors can be processed to remove up to 99.9% of blood, bone marrow and associated cells (Eagle et al. 2015). However, cut and shaped bone is not suitable for some orthopaedic procedures and some orthopaedic surgeons do not wish to use irradiated bone; therefore in this report, a method has been developed in which whole femoral heads can be washed to remove donor blood and bone marrow components. Processing results in excess of 99% bone marrow component removal—soluble protein, haemoglobin and DNA; the procedure is performed inside a closed system, thereby eliminating the need for terminal sterilisation by irradiation. In addition, uniaxial testing demonstrated no difference in compressive strength between washed and unwashed bone. We suggest that this washed bone may be capable of improving incorporation after grafting without disturbing biomechanical properties of the graft.





Similar content being viewed by others
References
Arts CJJ, Verdonschot N, Burma P, Schreurs BW (2006) Larger bone graft size and washing of bone grafts prior to impaction enhances the initial stability of cemented cups. Acta Orthop 77:227–233
Board TN (2007) Impaction grafting in revision hip surgery. MD thesis, University of Manchester
Eagle MJ, Man J, Rooney P, Hogg P, Kearney JN (2015) Assessment of an improved bone washing protocol for deceased donor human bone. Cell Tissue Bank 16(1):83–90
Hirn M, Laitinen M, Vuento R (2003) Pulse lavage washing in decontamination of allografts improves safety. Chir Org Mov 88:149–152
Ibrahim T, Qureshi A, McQuillan TA, Thomson J, Galea G, Power RA (2012) Intra-operative washing of morcellised bone allograft with pulse lavage: how effective is it in reducing blood and marrow content? Cell Tissue Bank 13(1):157–165
Lomas R, Drummond O, Kearney JN (2000) Processing of whole femoral head allografts: a method for improving clinical efficacy and safety. Cell Tissue Bank 1:193–200
Smith CA, Richardson N, Eagle MJ, Rooney P, Board T, Hoyland JA (2015) The use of a novel bone allograft wash process to generate a biocompatible, mechanically stable and osteoinductive biological scaffold for use in tissue engineering. J Tissue Eng Regen Med 9(5):595–604
Smith CA, Board TN, Rooney P, Eagle MJ, Richardson SM, Hoyland JA (2017) Human decellularized bone scaffolds from aged donors show improved osteoinductive capacity compared to young donor bone. PLoS ONE 12(5):e0177416
Tomford WW (1995) Transmission of disease through transplantation of musculoskeletal allografts. J Bone Jt Surg 77:1742–1754
van der Donk S, Weemink T, Burma P, Aspenberg P, Sloof TJ, Schreurs BW (2003) Rinsing morselised allografts improves bone and tissue ingrowth. Clin Orthop Relat Res 408:302–310
Voor MJ, Madsen R, Malkani A, Togawa D, Bauer TW (2008) Impaction grafting for femoral component revision in a goat model using washed morselized cancellous allograft. Orthopaedics 31:443
Yates P, Thomson J, Galea G (2005) Processing of whole femoral head allografts: validation methodology for the reliable removal of nucleated cells, lipid and soluble proteins using a multi-step washing procedure. Cell Tissue Bank 6:277–285
Acknowledgements
We acknowledge support of this work from the NHSBT Tissue and Eye Services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eagle, M.J., Man, J., Rooney, P. et al. Assessment of a closed wash system developed for processing living donor femoral heads. Cell Tissue Bank 18, 547–554 (2017). https://doi.org/10.1007/s10561-017-9664-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9664-z