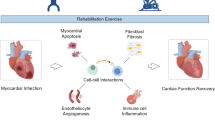
Abstract
Background
AMPK is considered an important protein signaling pathway that has been shown to exert prominent cardioprotective effects on the pathophysiological mechanisms of numerous diseases. Following myocardial infarction, severe impairment of cardiac function occurs, leading to complications such as heart failure and arrhythmia. Therefore, protecting the heart and improving cardiac function are important therapeutic goals after myocardial infarction. Currently, there is substantial ongoing research on exercise-centered rehabilitation training, positioning exercise training as a significant nonpharmacological approach for preventing and treating numerous cardiovascular diseases.
Objective
Previous studies have reported that exercise can activate AMPK phosphorylation and upregulate the AMPK signaling pathway to play a cardioprotective role in coronary artery disease, but the specific mechanism involved remains to be elucidated.
Conclusion
This review discusses the role and mechanism of the exercise-mediated AMPK pathway in improving postinfarction cardiac function through existing studies and describes the mechanism of exercise-induced myocardial repair of AMPK from multiple perspectives to formulate a reasonable and optimal exercise rehabilitation program for the prevention and treatment of myocardial infarction patients in the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI), a disease with a high incidence of cardiovascular disease, presents a pressing challenge in the diagnosis and treatment of noncommunicable diseases. The annual increase in incidence and the diagnosis of MI at younger ages underscore the urgency of addressing this issue [1]. Percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), as conventional treatment modalities, can effectively restore coronary artery blood circulation, reestablish reperfusion blood flow, and stabilize postoperative patient mortality at a low level (0.23%) [2, 3]. However, according to the statistics of INTERHEART, the prevalence of depression after acute myocardial infarction (AMI) in China is 21.66%, and the total mortality rate of postinfarction patients is 5.9%, 6.9%, and 7.6% at 30, 60, and 90 days, respectively, threatening the health of the whole population. These findings undoubtedly impose great economic and medical burdens on global public health treatment organizations [4]. Previous studies on the complex pathomechanisms of myocardial infarction have shown that myocardial apoptosis due to acute/chronic ischemia, inflammatory responses, imbalance of oxidative and antioxidant systems, and mitochondrial dysfunction due to internal environmental disturbances exacerbates postinfarction ventricular pathologic remodeling and deterioration of cardiac function [5,6,7,8]. Preventing these hazardous pathogenic factors and inhibiting adverse processes are crucial for myocardial infarction salvage and rehabilitation.
Exercise: Healthy Lifestyle to Disease Treatment
With the rapid development of social pace and people’s lifestyles and dietary changes, the positive effects of exercise training on physical fitness, psychological regulation, weight control, and other positive effects have become a national consciousness, and a variety of “exercise” programs have been pushed into the craze. Various exercise programs have gained popularity, driven by practical observations of rehabilitation patients, clinical data, and experimental studies, including animal in vivo models simulating exercise training. The positive impact of exercise training on human health and well-being has been substantiated through these studies [9]. For instance, exercise training serves as a highly effective preventive measure, reducing the risk of cancer [10, 11]. It is an excellent alternative to primary care medications for older adults with depression [12] and is an excellent means of preventing pregnancy-related disorders and prenatal and postpartum depression during pregnancy [13]. According to data from studies of coronary artery disease, regular cardiac exercise produces new cardiomyocytes at a rate of approximately 7.5% per year, which is 4.6 times greater than that in sedentary populations[14]. Long-term follow-up over > 36 months has shown a significant reduction in cardiovascular mortality as a result of exercise-based cardiac rehabilitation[15]; moreover, according to global surveys of noncommunicable disease data, nearly 9% of premature deaths are attributed to physical inactivity, and exercise-based rehabilitation reduces overall mortality in patients with coronary artery disease by 36–63%[16, 17]. Earlier research demonstrated the regenerative effects of exercise on myocardial cells and tissues, crucial for protecting coronary arteries from ischemic injury. Exercise induces a robust myocardial response in the infarct border zone and stimulates endogenous cardiomyocyte production in ischemia-treated mouse hearts [14]. As early as 1999, for the first time, Yamashitaet’s research team found that exercise reduced the infarct size by nearly 60% [18] in myocardial infarction mice; moreover, this exercise-triggered cardioprotection is activated prior to the onset of the disease, and it continues to be activated through far-reaching recovery from the disease [19, 20]. Mice trained with exercise before ischemic treatment, involving coronary ligation and coronary recanalization after ischemia, exhibited increased myocardial tolerance to ischemia and activation of antioxidant defense factors [21]. Moreover, evidence in medicine explains that exercise excites sympathetic nerves, inhibits parasympathetic nerves, increases catecholamine concentrations, and strengthens myocardial contractility, which slows down the pathological remodeling of the heart muscle after myocardial infarction [22]. An increasing number of patients with coronary artery disease obtain physical protection from exercise training; however, due to the complex pathology of coronary artery disease, exercise training is limited by exercise time, exercise intensity and exercise mode, and the mechanism of myocardial protection during exercise still needs perfecting. Adenylate-activated protein kinase (AMPK), a pivotal regulator of energy metabolism, mediates multiple molecular signaling pathways to protect the body’s energy consumption during myocardial ischemia and hypoxia via the same mechanism of myocardial protection, and exploring the signaling mechanism involved in exercise is important for coronary protection.
AMPK Structure and Activation
AMPK exists in various eukaryotic cells as a heterotrimer composed of α, β, and γ subunits, with an upstream kinase in the α subunit of the Thr172 residue necessary for AMPK activation [23]. The regulation of its activity is dependent on a change in the ratio of adenosine monophosphate (AMP) and adenosine-5’-diphosphate (ADP) as a way to regulate the phosphorylation of the Thr172α catalytic subunit by the upstream kinase liver kinase B1 (LKB1) and Ca2+/CaM-dependent protein kinase (CaMKKβ) [24]. In contrast, the γ subunit contains two pairs of CBS structural domains that bind AMP and ATP and serve as the primary adenosine-5’-triphosphate (ATP) binding domains under normal energy metabolism conditions [25]. AMPK is activated early during energy acquisition and transports membrane vesicles containing glucose transporter type 4 (GLUT4) to the sarcolemmal membrane to accelerate cellular glucose uptake, phosphorylate, and inhibit acetyl coenzyme A carboxylation (ACC) to regulate lipids, and inhibit fatty acid synthesis to regulate energy homeostasis [26, 27]. Hypertrophic adiposity, insulin resistance, and a strong inflammatory response were observed in AMPK knockout mice [28]. When mice were fed β-guanosine propionic acid (GPA), an analog capable of inducing an increase in the AMP/ATP ratio and AMPK activity, the associated increase in mitochondrial enzyme activity led to active mitochondrial biogenesis, whereas there was no effect on AMPK activity or mitochondrial biogenesis-related mitochondrial enzyme activity in transgenic mice with a dominant-negative mutation in AMPK [29]. The α2, β2, γ1, and γ2 isoform forms are most abundantly expressed in regulatory relationships in the heart, especially in ischemic myocardial tissues, where the AMPK γ1 isoform complex accounts for 70% of the cardiac AMPK activity; thus, progressive ventricular hypertrophy due to mutations in the γ2 subunit can be fatal in infants and children when mutations in the γ2 subunit result in progressive ventricular hypertrophy [30, 31]. AMPK serves as an energy sensor, effectively coordinating tissue metabolism to maintain intracellular environmental homeostasis under stress conditions. Its high sensitivity to exercise provides insights into the relationships between the cardioprotective effects of exercise on postinfarction oxidative damage, inflammatory attack and mitochondrial dysfunction and between AMPK signaling molecules. Understanding these relationships offers multiple perspectives on clinical therapeutic targets, drug development, and multifaceted ideas. Although the exact mechanisms underlying myocardial infarction are not fully understood, oxidative stress, inflammation, and mitochondrial dysfunction are involved in this process.
AMPK in Exercise Contraction
In earlier studies, AMPK has been proposed to mimic the effects of exercise [32], which means that the pharmacological effects of AMPK in disease can be realized through exercise activation. This approach aims to transform exercise from a “healthy lifestyle” to a “nonpharmacological therapeutic tool”. Skeletal muscle exercise induces various effects, marked by a significant increase in muscle energy turnover and alterations in nucleotide status. During exercise, ADP, a product of ATP hydrolysis, is rapidly converted to AMP by the adenylate kinase reaction, and the intracellular AMP/ATP ratio increases. This energetic stress causes AMP to bind to the CBS structural domain of the γ-subunit of AMPK to activate AMPK, promoting the phosphorylation of AMPK at the α-subunit of Thr172, which is capable of linearly increasing its activity depending on the intensity of the exercise by more than 100-fold, and this change is accompanied by a change in nucleotide status [33,34,35,36]. Western immunoblotting experiments revealed the activation of key signaling pathways, including the AMPK (Thr172) pathway, and the phosphorylation of the AMPK substrate acetyl coenzyme A carboxylic acid (ACC; S79) site in muscle after both in situ contraction stimulated by the sciatic nerve and treadmill exercise [37]. However, the different isomeric forms show different endpoints during exercise contractions depending on differences in exercise intensity and duration, with an increase in AMPKα2 activity observed at 70% VO2max exercise intensity for only 20 min, whereas AMPKα1 does not show significant changes in this exercise pattern and is activated only by intense exercise or strong physical body external electrical stimulation [38, 39].
In cardiac metabolism, glucose uptake, fatty acid uptake, and protein synthesis are important for the repair of damaged cardiac tissues. In ischemic and hypoxic myocardium, activated AMPK drives the translocation of GLUT-4 to increase glucose uptake in myocardial tissues, but, interestingly, the specific knockout of AMPKβ1β2 in mouse muscle tissues did not reverse the accelerated degradation of ATP and the concomitant decrease in glucose uptake in skeletal muscle during exercise, even with exercise, which seems to demonstrate that AMPK plays a central role in the regulation of homeostasis [40,41,42]. In contrast, when AMPKα was absent or severely inactivated, the total phospholipoprotein content of mice in the AMPKα-deficient group was still 20% higher than that of mice in the wild group, although it could be reduced by exercise. Meanwhile, the ratio of phosphorylated AMPK Thr172: total AMPKα in AMPK-deficient mice was approximately 65% lower compared with that in wild mice, and the cardiac endoplasmic reticulum Ca2+-ATPase 2a (SERCA2a) was reduced by approximately 37%. That is, exercise can trigger cardioprotective mechanisms in the presence of cardiac dysfunction, but only with the involvement of AMPK phosphorylation [43].
Different isoforms activated by the sensitivity of AMPK to exercise and the variety of exercise forms play different functional roles and are important for coronary and myocardial protection.
Oxidative Stress in AMPK
In CVD, the relationship between AMPK and oxidative stress involves mutual regulation and constraints. Oxidative stress can modulate the activity of AMPK, and, reciprocally, activated AMPK plays a crucial role in upregulating the expression of endogenous antioxidant genes, serving as a defense mechanism against oxidative damage primed by reactive oxygen species (ROS). Simultaneously, activated AMPK stimulates peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), contributing to mitochondrial biogenesis [30, 44]. Following a myocardial infarction, the myocardium undergoes ischemic and hypoxic stress, leading to the rupture of the mitochondrial respiratory chain. This process results in an accumulation of metabolites such as peroxides, superoxides, and hydroxyl radicals, collectively referred to as ROS, and a depletion of antioxidants [22]. This imbalance in oxidative and antioxidative processes triggers oxidative stress, causing damage to cellular membrane lipids and mitochondrial DNA. This, in turn, disrupts the integrity of cardiomyocytes, increases cellular permeability, and exacerbates cellular damage and apoptosis [25]. Exogenous AMPK activators significantly increase the expression of antioxidant factors, including catalase (CAT), superoxide dismutase (SOD) 1, SOD2, and uncoupling protein 2 (UPC2), and inhibit ROS production during hypoxia and reoxygenation in H9c2 cells in vivo [45, 46]. Specifically, under stressor stimulation, AMPK is able to respond sensitively to oxidative damage caused by the myocardium by blocking the production of superoxide from mitochondria or nicotinamide adenylate dinucleotide phosphate oxidase (Nox) while directly driving the activation of downstream antioxidant genes and promoting the migration and aggregation of nuclear factor erythroid-2-related factor 2 (NRF2) into the nucleus [47,48,49]. NRF2 is considered a nonnegligible major regulator of antioxidant defense that prevents cells from oxidative damage [50] and may become an important therapeutic strategy to prevent oxidative stress [51].
AMPK and Oxidative Stress During Exercise
Resveratrol, as an AMPK activator, induces AMPK activation by significantly reducing the level of NADPH oxidase, inhibiting ROS production, and increasing myocardial antioxidant enzyme activity [52]. This effect contributes significantly to the amelioration of myocardial injury. Interestingly, exercise training demonstrates similar myocardial protective effects as resveratrol, acting as an effective agonist of AMPK [53]. Swimming exercise activates SIRT1/AMPK/NRF2-mediated lipid metabolism and prevents the secretion of substrates involved in oxidative stress [54]. Angiotensin II (AngII) in the vasculature also regulates ROS production because an increase in AngII in the infarcted myocardium is accompanied by an increase in ROS content and NADPHase activity; however, after exercise training intervention, with the activation of AMPK, AngII expression decreases, and NADPHase activity is subsequently inhibited, preventing ROS biosynthesis [54]. This view is consistent with the findings of Geolotto et al., who concluded that the inhibition of NADPH oxidase activity reduces ROS production and that this effect is significantly enhanced by AMPK activators and significantly diminished by intervention with AMPK inhibitors [55]. In summary, exercise can function as an exogenous AMPK activator, exerting cardioprotective effects by enhancing antioxidative defense against myocardial infarction. This nonpharmacological research strategy proves highly effective, and its mechanism is closely associated with the AMPK signaling pathway.
Inflammatory Responses in AMPK
Systemic and local inflammation after myocardial infarction is widely accepted to accompany the onset to the end of MI. Coronary hypoxia disrupts vascular endothelial cell integrity and barrier function, leading to the infiltration of myocardial tissues with C-reactive protein aggregates in peri-infarct zones. Proinflammatory factors, such as serum tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are released [56], initiating an inflammatory cascade characterized by the recruitment of inflammatory cells, chemokines, and cell adhesion factors into the bloodstream [28, 57]. In this process, MI-triggered activation of nuclear factor-kappaB (NF-κB) and its subsequent nuclear translocation are considered to be the central effectors of inflammatory signaling, and this triggered inflammation promotes myocardial injury, repair, and scarring, leading to myocardial dysfunction and remodeling [58]. However, interestingly, the induced inflammatory response during the infarction process shows contradictory results, which in general means that inflammation during myocardial infarction is a double-edged sword for cardiac repair [57]. The inflammatory response occurs during myocardial ischemia and reperfusion and in the early phase of acute myocardial ischemia. Lipid metabolism restricts the inflammatory threat of proinflammatory cells to the myocardial myocardium by converting leukotriene B4 (LTB4) synthesis into lipid mediators [59, 60], and exogenous TNF-α stimulation has been suggested to increase rapamycin-induced myocardial autophagy and effectively prevent cardiomyocyte apoptosis[54]. However, in the later phase, persistent stimulation of NF-κB exacerbates reactive oxygen intermediates (ROIs) and nitric oxide (NO) production, and the myocardium is subjected to intense inflammatory attack with simultaneous activation of oxidative stress; moreover, the inflammatory response transforms itself into a key factor in accelerating myocardial dysfunction [60]. Liraglutide can treat myocardial inflammatory responses caused by metabolic disorders and mitochondrial dysfunction due to its activation of AMPK protein activity, which inhibits the inflammatory expression of interleukin 1β in cardiomyocytes [61], leveraging the classical anti-inflammatory properties of AMPK [54, 62].
AMPK and Inflammation in Exercise
The anti-inflammatory effects of exercise were revealed as early as the 1980s [63]. According to previous studies, exercise-induced AMPK signaling significantly ameliorates inflammation induced by high-fat factors [64]. Both aerobic and booster training promote the release of anti-inflammatory cytokines (IL-10), inhibit TNF-α secretion, and ameliorate cardiac fibrosis to reduce pathological myocardial remodeling [65, 66]. Although there is little direct evidence of the anti-inflammatory effects of exercise in relation to the AMPK mechanism, the evidence is summarized that exercise significantly promotes the release of the anti-inflammatory factor IL-10 and the interleukin IL-1 receptor antagonist and inhibits the secretion of TNF-α while blocking the inflammatory response of the Toll-like receptor pathway.
Mitochondrial Dysfunction in AMPK
Mitochondria play a crucial role in providing ATP energy, serving as the foundation for various tissues and organs to fulfill their metabolic functions [67]. Mitochondria are regarded as networked regulatory systems by virtue of their extraordinary morphological and structural plasticity. Regardless of physiological renewal and development or defense resistance under stressor stimuli, mitochondria achieve self-balancing and quality control through biogenesis, fission, fusion, and self-phagocytosis in response to cellular and environmental demands. In the heart, cardiomyocytes boast a robust mitochondrial network, constituting up to 30% of the heart volume. Oxidative phosphorylation within these mitochondria generates the energy necessary to sustain the high-density function of the heart. However, after myocardial infarction, ATP synthesis is impeded by continuous hypoxia and disruption of nutrient supply to cardiomyocyte tissues [68, 69]. At the same time, aerobic metabolism is impaired due to hypoxia in cardiomyocytes, and the mitochondrial environment is disrupted, leading to an imbalance in the acid‒base balance. This imbalance results in changes to acid‒base equilibrium, alterations in intramembrane permeability, Ca2+ entry into mitochondria, damage to the electron transport chain, and an excess of mitochondrial ROS and calcium overload. These factors collectively suppress normal biological functions [22, 70]. Interestingly, PGC-1α, a key regulator of mitochondrial biogenesis and a downstream AMPK protein pathway, can directly phosphorylate the PGC-1α protein at threonine 177 and serine 538, stimulating mitochondrial biogenesis and contributing to energy production [71,72,73]. Specific knockdown of the AMPKα subunit induces mitochondrial biogenesis as well as functional deficits [74]. An increasing number of studies have revealed the importance of AMPK in the regulation of mitochondrial homeostasis. AMPK is important for the regulation of mitochondrial homeostasis [75, 76].
AMPK and Mitochondrial Dysfunction in Exercise (Fig. 1)
The remarkable structural plasticity of mitochondria empowers them to modify their network structure through exercise training, resulting in a substantial increase of approximately 40–50% in total mitochondrial proteins. This alteration significantly enhances mitochondrial antioxidant capacity, attributed to Mn- and Cu-, Zn-superoxide dismutase and catalase, while also boosting overall mitochondrial function [73, 77]. The exogenous AMPK agonist AICAR significantly induces mitochondrial biogenesis in skeletal muscle after simulated exercise intervention [60]. The master transcriptional regulator PGC-1α, which plays a key bridging role, was identified [78]. Additionally, the increased PGC-1α and vascular endothelial growth factor (VEGF) action that accompanies exercise promotes the level of the core factor of neovascularization, HIF-1α mRNA, and this neovascularization also benefits from the role of AMPK activation [79, 80]. Animal studies have shown that significant activation of AMPK after acute exercise or exercise training is accompanied by an increase in ULK1 phosphorylation, and that activated AMPK is able to translocate ULK1 to mitochondria via phosphorylation at the serine 555 site [81, 82]. While specific deletion of AMPK in muscle tissue leads to mitochondrial damage, consistent with the mitochondrial dysfunction exhibited by ULK1-loss mice, it can be argued that exercise drives mitochondrial mitophagy, but this requires the involvement of the AMPK-ULK1 regulatory axis, and that the resulting autophagy is highly dependent on the intensity of the exercise and the AMPK activity [83].
In a mouse model of high-fat diet-induced diabetic cardiomyopathy, oxidative phosphorylation and the increase in the membrane potential were significantly enhanced through the AMPK/PGC-1α signaling pathway, ROS production and oxygen consumption were reduced, and myocardial damage caused by mitochondrial dysfunction and lipid metabolism disorders was ameliorated [84]. Similarly, during exercise, exercise activates the AMPK/PGC-1α signaling pathway, which promotes ATP production and increases mitochondrial biosynthesis [85], which implies that none of the genes involved in mitochondrial metabolism seem to be indispensable for the regulation of the PGC1 family through interactions with estrogen-associated receptors (ERRs) [86]. In addition, through fission and fusion associated with mitochondria after exercise, the minute expression of electron transport chain proteins of the tricarboxylic acid cycle machine is increased [2], which is similar to the role of AMPK and will lead to the exploration of new mechanisms linking exercise and AMPK.
Future Prospects
As previously mentioned, ischemia/reperfusion injury stemming from sustained ischemia/hypoxia and the subsequent restoration of reperfusion after infarction are significant contributors to the exacerbation of the infarction process. These processes involve oxidative stress, inflammation, and disrupted mitochondrial energy metabolism, which, if not addressed promptly, contribute to undesirable pathways. A large number of experiments have proven that AMPK signaling has a beneficial effect on reversing these undesirable pathways, and studies on the activation of the AMPK signaling pathway through exercise have also been reported. Research has also shown that this approach is undoubtedly beneficial to patients, regardless of the economic pressure on patients and recovery after intervention. Moreover, from the perspective of clinical care, in-depth studies of exercise-mediated rehabilitation mechanisms also provide nursing staff with a clearer theoretical support for patients to carry out disease and exercise rehabilitation from empty “empiricism” to solid knowledge of rehabilitation. The change from empty “empirical” to solid “theoretical perception” of the knowledge of disease and exercise rehabilitation for patients is clinically feasible and beneficial to patients. However, the specific protein pathways and targets through which exercise-mediated AMPK improves postinfarction cardiac function have yet to be studied in depth. Additionally, the safety of exercise training has not been definitively guaranteed in practical terms. Therefore, a thorough assessment of the operation and preparation of emergency plans for unforeseen safety incidents should precede exercise rehabilitation. Finally, this review aimed to illustrate the significance of nonpharmacological forms of exercise training in mediating AMPK phosphorylation on cardiac function after myocardial infarction, to further promote the value of cardiac rehabilitation and clinical care compliance and to improve the prognosis and quality of life of patients with myocardial infarction.
Availability of Data and Materials
All the information in this article is open and transparent.
References
China Cardiovascular Health and Disease Report 2022 Summary. China Circul Magazine. 2023;38(06):583–612.
Campos JC, Marchesi Bozi LH, Krum B, et al. Exercise preserves physical fitness during aging through AMPK and mitochondrial dynamics. Proc Natl Acad Sci U S A. 2023;120(2):e2204750120. https://doi.org/10.1073/pnas.2204750120.
Diao K, Wang D, Chen Z, et al. Rationale and design of a multi-center, prospective randomized controlled trial on the effects of sacubitril-valsartan versus enalapril on left ventricular remodeling in ST-elevation myocardial infarction: the PERI-STEMI study. Clin Cardiol. 2021;44(12):1709–17. https://doi.org/10.1002/clc.23744.
Long Z, Liu W, Zhao Z, et al. Case fatality rate of patients with acute myocardial infarction in 253 chest pain centers - China, 2019–2020. China CDC Wkly. 2022;4(24):518–21. https://doi.org/10.46234/ccdcw2022.026.
Ashraf MI, Ebner M, Wallner C, et al. A p38MAPK/MK2 signaling pathway leading to redox stress, cell death and ischemia/reperfusion injury. Cell Commun Signal. 2014;12:6. https://doi.org/10.1186/1478-811X-12-6.
Xiang M, Lu Y, Xin L, et al. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev. 2021;2021:6614009. https://doi.org/10.1155/2021/6614009.
Hernandez-Resendiz S, Chinda K, Ong SB, et al. The role of redox dysregulation in the inflammatory response to acute myocardial ischaemia-reperfusion injury - adding fuel to the fire. Curr Med Chem. 2018;25(11):1275–93. https://doi.org/10.2174/0929867324666170329100619.
Zhou H, Wang J, Zhu P, et al. Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal. 2018;45:12–22. https://doi.org/10.1016/j.cellsig.2018.01.020.
Brett JO, Arjona M, Ikeda M, et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. NatMetab. 2020;2(4):307–17. https://doi.org/10.1038/s42255-020-0190-0.
Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15(12):731–43. https://doi.org/10.1038/s41569-018-0065-1.
Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-Dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–62. https://doi.org/10.1016/j.cmet.2016.01.011.
López-Torres Hidalgo J; DEP-EXERCISE Group. Effectiveness of physical exercise in the treatment of depression in older adults as an alternative to antidepressant drugs in primary care. BMC Psychiatry. 2019;19(1):21.https://doi.org/10.1186/s12888-018-1982-6.
Ribeiro MM, Andrade A, Nunes I. Physical exercise in pregnancy: benefits, risks and prescription. J Perinat Med. 2021;50(1):4–17. https://doi.org/10.1515/jpm-2021-0315.
Vujic A, Lerchenmüller C, Wu TD, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9(1):1659. https://doi.org/10.1038/s41467-018-04083-1.
Dibben G, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021;11(11):CD001800. https://doi.org/10.1002/14651858.CD001800.pub4.
Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–35. https://doi.org/10.1001/jama.2009.681.
Tucker WJ, Fegers-Wustrow I, Halle M, et al. Exercise for primary and secondary prevention of cardiovascular disease: JACC Focus Seminar 1/4. J Am Coll Cardiol. 2022;80(11):1091–106. https://doi.org/10.1016/j.jacc.2022.07.004.
Yamashita N, Hoshida S, Otsu K, et al. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189(11):1699–706. https://doi.org/10.1084/jem.189.11.1699.
Garza MA, Wason EA, Cruger JR, Chung E, Zhang JQ. Strength training attenuates post-infarct cardiac dysfunction and remodeling. J Physiol Sci. 2019;69(3):523–30. https://doi.org/10.1007/s12576-019-00672-x.
Bozi LH, Maldonado IR, Baldo MP, et al. Exercise training prior to myocardial infarction attenuates cardiac deterioration and cardiomyocyte dysfunction in rats. Clinics (Sao Paulo). 2013;68(4):549–56. https://doi.org/10.6061/clinics/2013(04)18.
Demirel HA, Powers SK, Zergeroglu MA, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol (1985). 2001;91(5):2205–2212. https://doi.org/10.1152/jappl.2001.91.5.2205.
Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardialoxygen demand in young prehypertensive subjects. Am J Hypertens. 2013;26(9):1093–102. https://doi.org/10.1093/ajh/hpt080.
Cardaci S, Filomeni G Fau - Ciriolo M R and Ciriolo M R. Redox implications of AMPK-mediated signal transduction beyond energetic clues.J Cell Sci. 2012;125(Pt 9):2115–2125. https://doi.org/10.1242/jcs.095216.
Oakhill JS, Steel R, Chen ZP, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332(6036):1433–5. https://doi.org/10.1126/science.1200094.
Xiao B, Heath R, Saiu P, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449(7161):496–500. https://doi.org/10.1038/nature06161.
Wu N, Zheng B, Shaywitz A, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell. 2013;49(6):1167–75. https://doi.org/10.1016/j.molcel.2013.01.035.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–41. https://doi.org/10.1038/ncb2152.
Villena JA, Viollet B, Andreelli F, et al. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53(9):2242–9. https://doi.org/10.2337/diabetes.53.9.2242.
Zong H, Ren JM, Young LH, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99(25):15983–7. https://doi.org/10.1073/pnas.252625599.
Kukidome D, Nishikawa T, Sonoda K, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55(1):120–7.
Li J, Coven DL, Miller EJ, et al. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am J Physiol Heart Circ Physiol. 2006;291(4):H1927–34. https://doi.org/10.1152/ajpheart.00251.2006.
Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134(3):405–15. https://doi.org/10.1016/j.cell.2008.06.051.
Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am JPhysiol. 1996;270(2 Pt 1):E299–304. https://doi.org/10.1152/ajpendo.1996.270.2.E299.
Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546(1):113–20. https://doi.org/10.1016/s0014-5793(03)00560-x.
Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H. Dissociation of AMPK activity and ACCbeta phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun. 2002;298(3):309–16. https://doi.org/10.1016/s0006-291x(02)02465-8.
Stephens TJ, Chen ZP, Canny BJ, et al. Progressive increase in human skeletal muscle AMPKalpha2 activity andACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282(3):E688–94. https://doi.org/10.1152/ajpendo.00101.2001.
Hoffman NJ, Parker BL, Chaudhuri R, et al. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015;22(5):922–35. https://doi.org/10.1016/j.cmet.2015.09.001.
Fujii N, Hayashi T, Hirshman MF, et al. Exercise induces isoform-specific increase in 5’AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273(3):1150–5. https://doi.org/10.1006/bbrc.2000.3073.
Hayashi T, Hirshman MF, Fujii N, et al. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifyingcoupling mechanism. Diabetes. 2000;49(4):527–31. https://doi.org/10.2337/diabetes.49.4.527.
Jørgensen SB, Wojtaszewski JF, Viollet B, et al. Effects of alpha-AMPKknockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19(9):1146–8. https://doi.org/10.1096/fj.04-3144fje.
Lee-Young RS, Griffee SR, Lynes SE, et al. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem. 2009;284(36):23925–34. https://doi.org/10.1074/jbc.M109.021048.
Russell RR 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277(2):H643–9. https://doi.org/10.1152/ajpheart.1999.277.2.H643.
Morissette MP, Susser SE, Stammers AN, et al. Exercise-induced increases in the expression and activity of cardiac sarcoplasmic reticulum calcium ATPase 2 is attenuated in AMPKα(2) kinase-dead mice. Can J Physiol Pharmacol. 2019;97(8):786–95. https://doi.org/10.1139/cjpp-2018-0737.
Picard M, Gentil BJ, McManus MJ, et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J Appl Physiol (1985). 2013;115(10):1562–1571. https://doi.org/10.1152/japplphysiol.00819.2013.
Holness MJ, Bulmer K, Smith ND, Sugden MC. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem J. 2003;369(Pt 3):687–95. https://doi.org/10.1042/BJ20021509.
Bo H, Jiang N, Ma G, et al. Regulation of mitochondrial uncoupling respiration during exercise in rat heart: role of reactive oxygen species (ROS) and uncoupling protein 2. Free Radic Biol Med. 2008;44(7):1373–81. https://doi.org/10.1016/j.freeradbiomed.2007.12.033.
Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med. 2012;52(9):1607–19. https://doi.org/10.1016/j.freeradbiomed.2012.01.025.
Rabinovitch RC, Samborska B, Faubert B, et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21(1):1–9. https://doi.org/10.1016/j.celrep.2017.09.026.
Joo MS, Kim WD, Lee KY, et al. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol Cell Biol. 2016;36(14):1931–42. https://doi.org/10.1128/MCB.00118-16.
Park SY, Jin ML, Ko MJ, Park G, Choi YW. Anti-neuroinflammatory effect of Emodin in LPS-stimulated microglia: involvement of AMPK/Nrf2 activation. Neurochem Res. 2016;41(11):2981–92. https://doi.org/10.1007/s11064-016-2018-6.
Wu S, Zou MH. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int J Mol Sci. 2020;21(14):4987. https://doi.org/10.3390/ijms21144987.
Guo S, Yao Q, Ke Z, et al. Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol. 2015;412:85–94. https://doi.org/10.1016/j.mce.2015.05.034.
Zhou SX, Zhou Y, Zhang YL, Lei J, Wang JF. Antioxidant probucol attenuates myocardial oxidative stress and collagen expressions in post-myocardial infarction rats. J Cardiovasc Pharmacol. 2009;54(2):154–62. https://doi.org/10.1097/FJC.0b013e3181af6d7f.
Misra A, Haudek SB, Knuefermann P, et al. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation. 2003;108(25):3075–8. https://doi.org/10.1161/01.CIR.0000108929.93074.0B.
Ceolotto G, Gallo A, Papparella I, al. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27(12):2627–2633. https://doi.org/10.1161/ATVBAHA.107.155762.
Altenhöfer S, Kleikers PW, Radermacher KA, et al. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci. 2012;69(14):2327–43. https://doi.org/10.1007/s00018-012-1010-9.
Zou Y, Chen Z, Sun C, et al. Exercise intervention mitigates pathological liver changes in NAFLD zebrafish by activating SIRT1/AMPK/NRF2 signaling. Int J Mol Sci. 2021;22(20):10940. https://doi.org/10.3390/ijms222010940.
Dewald O, Ren G, Duerr GD, et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164(2):665–77. https://doi.org/10.1016/S0002-9440(10)63154-9.
Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–73. https://doi.org/10.1161/CIRCRESAHA.111.243162.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65. https://doi.org/10.1038/nrcardio.2014.28.
Li L, Pan R, Li R, Niemann B, et al. Mitochondrial biogenesis and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation by physical activity: intact adipocytokine signaling is required. Diabetes. 2011;60(1):157–67. https://doi.org/10.2337/db10-0331.
Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–69. https://doi.org/10.1172/JCI97943.
Ren J, Xu X, Wang Q, et al. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammationin endotoxemia. J Mol Cell Cardiol. 2016;93:18–31. https://doi.org/10.1016/j.yjmcc.2016.02.002.
Chandrasekar B, Freeman GL. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997;401(1):30–4. https://doi.org/10.1016/s0014-5793(96)01426-3.
Bergeron R, Ren JM, Cadman KS, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281(6):E1340–6. https://doi.org/10.1152/ajpendo.2001.281.6.E1340.
Hou Y, Wei D, Bossila EA, et al. FABP5 Deficiency Impaired Macrophage Inflammation by Regulating AMPK/NF-κB Signaling Pathway. J Immunol. 2022;209(11):2181–91. https://doi.org/10.4049/jimmunol.2200182.
Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154–1162. https://doi.org/10.1152/japplphysiol.00164.2004.
Thygesen K, Alpert JS, Jaffe AS, White HD. Diagnostic application of the universal definition of myocardial infarction in the intensive care unit. Curr Opin Crit Care. 2008;14(5):543–8. https://doi.org/10.1097/MCC.0b013e32830d34b9.
Jendrach M, Mai S, Pohl S, Vöth M, Bereiter-Hahn J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA aftertransient oxidative stress. Mitochondrion. 2008;8(4):293–304. https://doi.org/10.1016/j.mito.2008.06.001.
Bagheri F, Khori V, Alizadeh AM, et al. Reactive oxygen species-mediated cardiac-reperfusion injury: mechanisms and therapies. Life Sci. 2016;165:43–55. https://doi.org/10.1016/j.lfs.2016.09.013.
Tian L, Cao W, Yue R, et al. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J Pharmacol Sci. 2019;139(4):352–60. https://doi.org/10.1016/j.jphs.2019.02.008.
Almannai M, El-Hattab AW, Ali M, Soler-Alfonso C, Scaglia F. Clinicaltrials in mitochondrial disorders, an update. Mol Genet Metab. 2020;131(1–2):1–13. https://doi.org/10.1016/j.ymgme.2020.10.002.
Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22. https://doi.org/10.1073/pnas.0705070104.
Jung TW, Park HS, Choi GH, Kim D, Lee T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J Biomed Sci. 2018;25(1):27. https://doi.org/10.1186/s12929-018-0431-7.
Tuon T, Souza PS, Santos MF, et al. Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson’s Disease. Oxid Med Cell Longev. 2015;2015:261809. https://doi.org/10.1155/2015/261809.
Kesherwani V, Chavali V, Hackfort BT, Tyagi SC, Mishra PK. Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front Physiol. 2015;6:124. https://doi.org/10.3389/fphys.2015.00124.
Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R505–11. https://doi.org/10.1152/ajpregu.00208.2003.
Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11(1):102. https://doi.org/10.1038/s41467-019-13668-3.
Geng T, Li P, Okutsu M, et al. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 2010;298(3):C572–9. https://doi.org/10.1152/ajpcell.00481.2009.
Taylor CW, Ingham SA, Hunt JE, et al. Exercise duration-matched interval and continuous sprint cycling induce similar increases in AMPK phosphorylation, PGC-1α and VEGF mRNA expression in trained individuals. Eur J Appl Physiol. 2016;116(8):1445–54. https://doi.org/10.1007/s00421-016-3402-2.
Schwalm C, Jamart C, Benoit N, et al. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J. 2015;29(8):3515–26. https://doi.org/10.1096/fj.14-267187.
Tian W, Li W, Chen Y, et al. Phosphorylation of ULK1 by AMPK regulates translocation of ULK1 to mitochondria and mitophagy. FEBS Lett. 2015;589(15):1847–54. https://doi.org/10.1016/j.febslet.2015.05.020.
Spaulding HR, Yan Z. AMPK and the adaptation to exercise. Annu Rev Physiol. 2022;84:209–27. https://doi.org/10.1146/annurev-physiol-060721-095517.
Wang SY, Zhu S, Wu J, et al. Exercise enhances cardiac function by improving mitochondrial dysfunction and maintaining energy homoeostasisin the development of diabetic cardiomyopathy. J Mol Med (Berl). 2020;98(2):245–61. https://doi.org/10.1007/s00109-019-01861-2.
Kim MB, Kim T, Kim C, Hwang JK. Standardized Kaempferia parviflora extract enhances exercise performance through activation of mitochondrial biogenesis. J Med Food. 2018;21(1):30–8. https://doi.org/10.1089/jmf.2017.3989.
Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–24. https://doi.org/10.1016/S0092-8674(00)80611-X.
Funding
This work was supported by the National Natural Science Foundation of China [82160060, 82360086] and Guizhou Provincial Science and Technology Projects ZK [2022] YB669.
Author information
Authors and Affiliations
Contributions
Xiao di Zhang and Yi Zhao researched and organized the literature; designed the paper framework; drafted the paper; and revised the paper. Dafen Guo, Mingxian Luo, and Qing Zhang helped collect and collate the data. Li Zhang* and Deng-Shen Zhang* provided research funding, provided technical or material support, and provided guidance.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Consent to Participate
All authors agree to the publication of this manuscript.
Competing Interests
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Zhao, Y., Guo, D. et al. Exercise Improves Heart Function after Myocardial Infarction: The Merits of AMPK. Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-024-07564-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-024-07564-2