Abstract
Purpose
Patients with hyperlipidemia treated with statins remain at a residual cardiovascular (CV) risk. Omega-3 polyunsaturated fatty acids hold the potential to mitigate the residual CV risk in statin-treated patients, with persistently elevated triglyceride (TG) levels.
Method
We reviewed the current evidence on the use of icosapent ethyl (IPE), an omega-3 fatty acid yielding a pure form of eicosapentaenoic acid.
Results
REDUCE-IT reported a significant 25% reduction in CV events, including the need for coronary revascularization, the risk of fatal/nonfatal myocardial infarction, stroke, hospitalization for unstable angina, and CV death in patients on IPE, unseen with other omega-3 fatty acids treatments. IPE was effective in all patients regardless of baseline CV risk enhancers (TG levels, type-2 diabetes status, weight status, prior revascularization, or renal function). Adverse events (atrial fibrillation/flutter) related to IPE have occurred mostly in patients with prior atrial fibrillation. Yet, the net clinical benefit largely exceeded potential risks. The combination with other omega-3 polyunsaturated fatty acids, in particular DHA, eliminated the effect of EPA alone, as reported in the STRENGTH and OMEMI trials. Adding IPE to statin treatment seems to be cost-effective, especially in the context of secondary prevention of CVD, decreasing CV event frequency and subsequently the use of healthcare resources.
Conclusion
Importantly, IPE has been endorsed by 20 international medical societies as a statin add-on treatment in patients with dyslipidemia and high CV risk. Robust medical evidence supports IPE as a pillar in the management of dyslipidemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While the cardiovascular (CV) risk incurred from long-standing dyslipidemia, hypertension, diabetes, and other conditions is firmly established, residual CV risk has only recently become of interest in the race to prevent or mitigate CV diseases (CVD). Residual CV risk refers to the risk of emergent CV events that lingers in patients offered standard medical care or in patients with CV risk factors [1, 2]. The concept of residual CV risk stems from clinical trials on lipid-lowering strategies, mainly statin treatments, and extends to the treatment of hypertension, diabetes, and other CV risk factors [3]. Recent evidence supports the initiation of high intensity lipid-lowering agents with ongoing statin therapy [4,5,6], in an effort to promote further lowering of low-density lipoprotein (LDL)-cholesterol, as well as other atherogenic lipoproteins, and to normalize triglyceride (TG) levels. In fact, the cardio-metabolic risk is based on the concept of risk continuum and must be managed in a holistic manner.
A panel of 10 physicians from the United Arab Emirates (UAE) and the United States of America (USA) convened to review available evidence on the use of omega-3 polyunsaturated fatty acids, and in particular icosapent ethyl (IPE), in the treatment of dyslipidemia and CV risk. Physicians were cardiologists and endocrinologists with expertise in the field of dyslipidemia management and CV protection. Opinions and clinical practice were exchanged in a structured discussion orchestrated by the main author. Recommendations were collected and disseminated among experts before consolidation in the current manuscript.
This manuscript summarizes the state-of-the-art evidence and includes the most recent literature about the use of IPE to mitigate residual CV risk and halt CVD progression. Between June 2021 and June 2023, several drafts were generated to discuss and include newly published papers. This work is a critical appraisal of IPE use in the clinic with a clear comparison with other forms of polyunsaturated fatty acids. The manuscript can serve as a complete compilation of data of IPE and evidence-based guidance for the management of (residual) CV risk.
Risk Factors and Risk Enhancers in Cardiovascular Disease
Are Patients with Atherosclerotic CVD, Hypertension, Kidney Disease, Diabetes, or Hyperlipidemia at Higher Risk for CVD?
The combination of specific comorbidities exacerbates CV risk, especially the clustering of largely modifiable risk factors. Abdominal obesity is a major modifiable risk factor tightly associated with insulin resistance, low HDL-cholesterol levels, high TG levels and inflammatory markers, which is usually referred to as atherogenic dyslipidemia. In patients with diabetes, the frequency of CV events was attenuated by 6% upon a decrease of 4 mmHg in systolic blood pressure, by 4% upon a 1 mmol/l (38.7 mg/dl) decrease in LDL-cholesterol and by 1.5% upon lowering of HbA1c by 0.9% [7]. There is also a quantitative association between kidney function (estimated glomerular filtration rate [eGFR]) and the risk of CVD; lower eGFR exponentially increases the CV risk [8, 9]. A direct linear relationship between hyperlipidemia, in particular elevated LDL-cholesterol levels, and the occurrence of CVD, has been well established [10, 11]. Importantly, cardiac events can occur in people with controlled LDL-cholesterol levels (between 2.8 and 3.4 mmol/l or 110 and 130 mg/dl) [12], which calls into question the definition of a “normal” LDL-cholesterol level. Epidemiological data from major statin clinical trials concluded that, despite LDL-cholesterol control, a 56% to 85% residual CV risk remains [13,14,15,16,17,18,19,20,21,22] in patients with hypertriglyceridemia [23]. In fact, CV risk amplifies with increasing TG levels up to 1.7 mmol/l (150 mg/dl) in patients with statin-controlled low LDL-cholesterol levels [18, 24]. Collectively, these conditions consort to exacerbate the CV risk and, even when treated, still entail a residual risk [12]. Eradicating CVD in the future warrants lifestyle changes and the use of emerging pharmacotherapies [25].
Guideline Definitions of CV Risk Categories
According to International Recommendations, How Do the Different Comorbidities Define CV Risk?
Risk categories have been defined and updated, and proposed CV risk scoring systems have been validated or refuted in different populations [26, 27], but some risk factors put the patient at undeniably high risk for CVD. The European Society of Cardiology/European Atherosclerosis Society have defined LDL-cholesterol treatment goals according to different categories of total CV risk [27], as depicted in Fig. 1.
Treatment goals for LDL-cholesterol across categories of total CV risk. ASCVD: atherosclerotic cardiovascular disease; BP, blood pressure; CKD, chronic kidney disease; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FH, familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; SCORE, Systematic Coronary Risk Estimation; T1DM, type-1 DM; T2DM, type-2 DM; TC, total cholesterol [28]. This image is reproduced from the European Heart Journal, Mach et al. [28], upon permission granted by Oxford University Press on behalf of the authors. Not to be reproduced without prior permission from Oxford University Press
What is the Standard of Care for Atherosclerotic CVD, Familial Hyperlipidemia, Hypertension, Kidney Disease, and Type II Diabetes, and How are these Conditions Prioritized?
The holistic management of all risk factors is recommended [27]. Statin therapy is recommended in all patients with established CVD, in patients with type-2 diabetes [29], and especially those with chronic kidney disease [30,31,32]. Antiplatelet therapy is essential in all patients with established CVD [33]. The presence of diabetes mellitus clearly steers the decision toward treating patients with comorbid conditions, such as hypertension, dyslipidemia, or advanced age. However, particularly patients with dyslipidemia and diabetes should be prescribed statins to reduce the risk of coronary heart disease and stroke [34, 35]. Patients with diabetes benefited from intensive statin therapy [36]. Primary prevention is essential to prevent CV events in the broader population based on careful assessment of risk factors, secondary prevention is key to impeding or delaying the onset of recurrent CVD events and complications.
Triglycerides as a Causal Risk Factor for CVD
What Blood Lipid Profile is Achieved by Long-Term Statin Treatment?
Highly robust evidence of the CV risk reduction upon long-term statin use [37] makes statins the drugs of choice in the treatment of dyslipidemia and the prevention of atherosclerotic CVD [12, 38], with a rather positive risk/benefit ratio even with the lowest LDL-cholesterol achieved on therapy [39]. Several studies suggest that lowering LDL-cholesterol results in a linear decline in major adverse CV events (MACE); however, all atherogenic components interact to predict residual CVD particularly despite statin therapy [40, 41]. This implies that, while lowering LDL-cholesterol remains at the forefront of dyslipidemia treatment, TG levels should also be monitored as they are considered as a risk enhancer.
Elevated Triglyceride Levels, Chronic Inflammation, and CVD: Is There an Undeniable Causality?
Epidemiological studies have shown that hypercholesterolemia and advancing age are both risk factors of atherosclerotic CVD; i.e., the greater burden of hypercholesterolemia either due to higher levels or earlier (longer duration) exposure, the earlier the onset of atherosclerotic CVD [42]. Worldwide TG levels are on the rise in different populations, and TG-rich lipoproteins (mostly apolipoprotein [Apo] C3) constitute a CV risk factor, even in the case of elevated HDL-cholesterol and ApoA1 [43]. A recent Mendelian randomization study showed that the reverse is true; LDL-cholesterol or TG-lowering genetic variants are associated with a similar reduction in CVD risk, potentially through ApoB, a common carrier between TG and LDL-cholesterol in the blood [44]. Patients who are not genetically protected against elevated TG or LDL-cholesterol levels would greatly benefit from early treatment initiation to reduce complications from hyperlipidemia. In fact, lowering LDL-cholesterol by 0.025 mmol/l (4.9 mg/dl) and TG by 0.27 mmol/l (24 mg/dl) has been shown to result in a substantial reduction in CV events. However, statins, despite decreasing LDL-cholesterol levels (sometimes aggressively to below 1.8 mmol/l or 70 mg/dl), still do not eradicate CV risk. This, coupled with the persistence of TG and other subsets of cholesterol-carrier lipoproteins, results in the persistence of a residual CV risk [45]. These studies serve to refocus attention on TG and position TG lowering as an important factor, alongside LDL-cholesterol, in the fight against atherosclerotic CVD.
There is a tight interplay between lipid metabolism and chronic inflammation. Atherosclerotic disease, in all its stages, presents with chronic low-grade inflammation [46,47,48]. Indeed, inflammation, through elevated blood C reactive protein (CRP) levels, is an established CVD risk enhancer [48, 49], as defined by international guidelines. CRP levels decrease upon treatment with statins [47, 50] and with omega-3 fatty acids [51]. The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) showed that high-sensitivity CRP (hsCRP) level was reduced by 39.9% as compared to placebo, which was statistically significant in patients who already achieved target LDL-cholesterol levels on statin therapy [52], in line with the literature [53]. Addition of IPE treatment to statin resulted in a reduction in major CV events in primary and secondary prevention settings, mainly CV death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization [54] as well as significant improvement of plaque regression [55,56,57].
Residual CV Risk: Definition, Pharmacological Management
How is Residual CV Risk Evaluated in Clinical Trials (Biomarkers Versus Clinical Manifestations)?
Residual CV risk is defined as an extremely high risk of CV events in patients already treated for CV risk factors or recurring/progressive atherosclerotic CVD [58], which might occur in other arterial territories (cerebral vascular accident, CVD, peripheral artery disease). Residual risk clinically manifests itself by the occurrence of a new or a repeated CV event in spite of achieving target LDL-cholesterol (< 1.8 mmol/l or 70 mg/dl), suggesting that the current therapeutic approach has failed to prevent onset or progression of CVD. This is driven by a plethora of factors, including biochemical markers (high TG and low HDL levels, elevated levels of lipoprotein(a), apolipoprotein B and CRP), metabolic syndrome, insulin resistance, or established diabetes [58].
What is the Existing Evidence for Mitigating Residual CV Risk in Statin-Treated Patients?
There is considerable heterogeneity in the outcomes of TG-lowering therapies that differ based on the pharmacological mechanism. As recently reviewed, fibrates have been extensively evaluated. The Veterans Affairs Cooperative Studies Program High-Density Lipoprotein Cholesterol Intervention (1999 VA-HIT) trial, the 2005 Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial, and the 2010 Action to Control Cardiovascular Risk in Diabetes (ACCORD)-Lipid trial reported a significant decrease in TG levels upon treatment with gemfibrozil, fenofibrate, and combined statin/fenofibrate, respectively [59]. However, in the FIELD trial, except in the subgroup of patients with elevated TG and low HDL-cholesterol levels, the risk of coronary events (death or nonfatal myocardial infarction) was not mitigated upon fibrate use [60]; and in the ACCORD-Lipid trial, 200 mg fibrates did not lead to reduction in the rate of fatal CV events, nonfatal myocardial infarction, or stroke [61]. The phase 3 Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study planned to investigate the effects of pemafibrate on risk of CV events in high-risk patients with type-2 diabetes, mild-to-moderate hypertriglyceridemia, and low levels of HDL-cholesterol treated with statins [62]. The primary endpoint was a composite of nonfatal MI, nonfatal ischemic stroke, coronary revascularization, and CV death. The PROMINENT study was discontinued early based on the recommendations of the Data Safety Monitoring Board (DSMB). Despite lowering TG levels, pemafibrate did not decrease the incidence of CV events compared to placebo. However, pemafibrate was associated with a higher incidence of adverse renal events and venous thromboembolism despite the similar overall incidence of serious adverse events between groups [63].
Niacin is no longer recommended in the guidelines for the management of hyperlipidemia, driven by the following evidence: TG levels were only marginally decreased upon treatment with niacin alone or in association with laropiprant in the 2011 Atherothrombosis Intervention in Metabolic Syndromes with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) trial [64] and the 2014 Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) [65], respectively.
Omega-3 fatty acids have been extensively studied since the original Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto (GISSI) Prevention study in 1999. In that study, treatment in post-myocardial infarction patients with omega-3 polyunsaturated fatty acids resulted in significant decrease in TG levels and a decreased risk of CV event [66]. The more recent Japan EPA Lipid Intervention Study (JELIS) trial utilizing modern preventive therapy, including statin, highlighted the beneficial effects of eicosapentaenoic acid (EPA) on TG lowering and significant CV risk reduction, even at lower doses. Additionally, the Randomized Trial for Evaluating Secondary Prevention Efficacy of Combination Therapy - Statin and Eicosapentaenoic Acid (RESPECT-EPA) study followed around 3900 patients with stable coronary artery disease, randomized to 1.8 g/day IPE versus no IPE, on top of statin treatment, for the primary endpoint of major adverse cardiac events (cardiovascular death, myocardial infarction, stroke, unstable angina requiring hospitalization, and revascularization) [67,68,69]. The RESPECT-EPA trial found that the primary outcome occurred in 10.9% of patients on IPE compared to 14.9% of control patients, with a trend toward statistical significance (P = 0.055). In addition, the secondary outcomes (sudden cardiac death, myocardial infarction, unstable angina, or coronary revascularization) occurred less frequently in the IPE group than in the control group (8.0% versus 11.3%, P = 0.031) [70].
Table 1 revisits the major outcomes of studies on omega-3 fatty acid use in mitigating CV risk.
How Does the Cardio-Protective Role of IPE Unfold? Results of REDUCE-IT
Several omega-3 fatty acids have been evaluated as TG-lowering agents, showing successful lowering of TG levels. However, only pure IPE was associated with proven and sustained CV benefits. In fact, mixtures of other low dose and high dose omega-3 fatty acids showed no significant CV benefit in terms of coronary heart disease, stroke, revascularization, or any major vascular event [78].
A landmark clinical trial, REDUCE-IT, confirmed the relevance of evaluating medications that further lower TG levels and simultaneously yield different outcomes; i.e., TG level reduction and mitigation of CV events. REDUCE-IT evaluated the effects of IPE on the composite CV outcome (CV death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and unstable angina requiring hospitalization). As shown in Table 2, compared to placebo, the proportion of patients with a CV event occurring in the 5 years following randomization was significantly lower by 25% [52]. Taken separately, CV outcomes were also significantly reduced in patients on IPE compared to those on placebo. A 35% reduction in revascularization was reported in REDUCE-IT, compared with a 24% reduction with stenting in the Norwegian Coronary Stent Trial (NORSTENT) trial, 22% in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial, and 12% in the Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab (ODYSSEY OUTCOMES) trial. The revascularization risk reduction with IPE (35%) was comparable to that reported with simvastatin versus placebo use in 1994 (37%) [79]. In addition to decreasing the number of overall CV events, IPE also resulted in significantly fewer second, third, and fourth events (i.e., continuous reduction of recurrent CV events), compared to placebo [80].
Importantly, the primary composite outcome was significantly reduced regardless of baseline TG levels whether modestly elevated (> 1.69 mmol/l or 150 mg/dl) or more severely elevated (> 2.26 mmol/l or 200 mg/dl); both groups equally benefited from substantial reduction in CV events with IPE group [80]. This means that TG is a marker of increased risk and the addition of IPE provides a substantial benefit regardless of TG level studied, which has led to the label indication for reduction in CV events for patients with elevated TG (> 1.69 mmol/l or 150 mg/dl).
Compared to placebo, all CV outcomes significantly improved with IPE use throughout the study, and both primary and secondary outcomes were sustained over at least 2 years. In REDUCE-IT, for every 1000 patients treated with IPE and followed over 5 years, 12 fewer CV deaths, 42 fewer myocardial infarctions, 14 fewer strokes, 76 fewer coronary revascularization interventions, 16 fewer hospitalization for unstable angina, and 159 fewer total primary outcome events were reported, compared to placebo-treated patients [80].
REDUCE-IT was further dissected according to population subgroups. Figure 2 below summarizes the effects of IPE treatment in patients at high CV risk, and with different comorbid conditions [81,82,83,84,85,86,87]. Recently, the effect of IPE was evaluated in post-hoc analyses of REDUCE-IT, examining the benefits of IPE in patients with different smoking history (current, former, and never smokers). The time to primary composite endpoint (CV death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina) was once again greatly prolonged in IPE-treated patients compared to placebo, regardless of smoking status [88].
IPE at 4 g daily confers CV benefits in patients with and without hyperlipidemia (from REDUCE-IT). A. IPE resulted in a reduction in the rate of total primary and secondary endpoints for patients with different baseline characteristics. B. Compared to placebo, there was an increase in time to first revascularization of at least 32% in patients treated with IPE C. IPE decreased the incidence of total stroke events. D. IPE use was associated with significant and consistent delay in the occurrence of CV events in all smoking status categories (A stand-alone post hoc analysis). Primary endpoint: composite of CV death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina. Key secondary endpoints: a composite of CV death, nonfatal myocardial infarction, or nonfatal stroke. High TG: TG ≥200 mg/dl. Low HDL-C: HDL-C ≤35 mg/dl. CV death was also displayed separately, given its importance as the ultimate benefit. BMI, body mass index; CABG, coronary artery bypass graft; CV, cardiovascular; HDL-C, High-density lipoprotein cholesterol; IPE, icosapent ethyl; MI, myocardial infarction; PCI, percutaneous coronary intervention; TG, triglycerides. [81,82,83,84,85,86,87]
Could the Mechanism of Action of IPE Explain the Substantial Magnitude of the Positive Outcomes with IPE Compared to Other Omega-3 Fatty Acids?
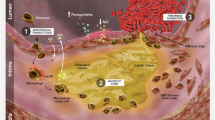
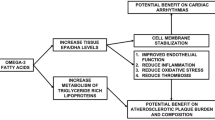
IPE is a substrate for intestinal lipase, which de-esterifies it to yield EPA. EPA diffuses into the intestinal epithelial cells, where it is re-esterified and packaged into chylomicrons that cross into the lacteals, to eventually join the circulation [89]. EPA incorporates in the lipid bilayer of the plasma membrane, without disrupting its architecture, cholesterol distribution or normal fluidity. Figure 3 summarizes the molecular effects of IPE, which yield its CV effects through potential antioxidant properties and promoting anti-inflammatory processes. Conversely, docosahexaenoic acid (DHA), another major marine-derived omega-3 fatty acid, which also incorporates in the plasma membrane, increases its fluidity and modulates lipid domains, with reduced antioxidant activity [90].
Mechanisms of action of IPE, driving its effects on atherosclerotic plaque. EPA incorporates in the phospholipid bilayer, promoting anti-inflammatory processes, decreasing lipid oxidation, immune cell recruitment, and pro-inflammatory processes [45, 57]. Preclinical in vitro studies report that EPA reduces the expression of pulmonary ACE and ICAM-1, upregulated in response to pro-inflammatory IL-6, preserving vascular endothelial function [90, 91]. AA, arachidonic acid; ACE, angiotensin-converting enzyme; EPA, eicosapentaenoic acid; hsCRP, high sensitivity C reactive protein; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; IPE, icosapent ethyl; Lp-PLA2, lipoprotein-associated phospholipase A2; MMPs, matrix metalloproteinases; NO, nitric oxide
In contrast to other omega-3 trials with specific omega-3 formulations, the magnitude of benefits in CV risk seen in REDUCE-IT has not been replicated. It can be concluded that IPE is the specific agent behind this CV benefit that was not reported in the STRENGTH trial using omega-3 carboxylic acid combination.
The STRENGTH trial of a specific omega-3 carboxylic acid composition (EPA + DHA) did not lead to improved CV outcomes, compared to placebo and was discontinued prematurely [92]. In the Omega-3 Fatty acids in Elderly with Myocardial Infarction (OMEMI) trial that was a smaller study, administering a mixture of EPA and DHA to elderly patients’ post-myocardial infarction did not improve CV outcomes compared to placebo [93]. Findings from REDUCE-IT , as well as from other trials evaluating omega-3 fatty acids, were scrutinized in an attempt to identify the reason behind discrepancy in CV outcomes upon treatment with the different omega-3 fatty acid agents. From a pharmacological standpoint, this would appear to be a comparison between pure IPE therapies versus therapies that contain a mixture of EPA and DHA.
Could the Use of Mineral Oil in the Placebo Arm Explain the Magnitude of Benefits?
Some have argued that the substantially positive results from REDUCE-IT might be attributed to a negative CV effects of the mineral oil-based placebo rather than the beneficial effects of IPE [94]. The safety of pharmaceutical grade mineral oil was investigated and its use as placebo in clinical trials was found to be acceptable and not biasing trial outcomes [95].
Despite robust evidence, controversy has been raised about the interpretation of REDUCE-IT. However, statistical analysis reveals that the effect of IPE cannot be due to chance given the robust study design and power to detect statistical difference. Second, confounding factors, such as the use of mineral oil in the placebo arm has also been rigorously analyzed; only small absolute changes in inflammatory and lipid biomarkers other than TG were found, and these were not associated with CV outcomes in the study [95, 96]. In addition, the 2020 review did not identify any consistent trend in lipid levels or inflammatory markers in patients given mineral oil. Furthermore, an analysis of REDUCE-IT by baseline statin use (hydrophilic versus hydrophobic) found consistent benefits, again arguing against any interaction between mineral oil and statins [97]. This reassurance on the robust conclusions obtained by REDUCE-IT is further backed up by results from the JELIS trial, which did not use mineral oil as placebo [54]. Additionally, very recent research reported that IPE inhibits LDL-cholesterol oxidation, compared to mineral oil and DHA in vitro [98]. In particular, the rate of LDL-cholesterol oxidation was not affected by mineral oil (P < 0.001), while EPA significantly inhibited LDL-cholesterol oxidation compared to vehicle (P < 0.001). DHA exerted its anti-oxidant activity only for 2 h, and to a lesser level than EPA (P < 0.05). The longer-term antioxidant potential of EPA may contribute to decreased incidence of CV events [98]. Another in vitro study demonstrated that the antioxidant activity of mineral or corn oil was inexistent, even at supra-pharmacological doses, underscoring that the antioxidant activity is actually attributable to IPE and not placebo choice [99].
Does Elevated Serum EPA Level Reduce CV Risk?
Atherosclerotic plaque remodeling has been used for decades as a surrogate for CV outcomes, and a proof for the biological effects of statins (the SATURN trial [100]) and of proprotein convertase subtilisin/kexin type-9 (PCSK9) inhibitors (the GLAGOV trial [101]). The Combination Therapy of Eicosapentaenoic acid and Pitavastatin for Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography (CHERRY) trial results support that prescription EPA reduces coronary plaque volume [55]. The more recent Effect of Vascepa on Improving Coronary Atherosclerosis in People with High Triglycerides Taking Statin Therapy (EVAPORATE) trial also confirms that IPE decreases plaque volume by 9% (versus an increase by 11% in the placebo arm) and improves plaque composition [57, 102]. Looking at these mechanistic studies, which report on atherosclerotic plaque regression with IPE, it is abundantly clear that neither study design nor the comparator placebo arm could explain the difference in outcome between combination EPA and DHA treatment or EPA alone, except the beneficial effect of IPE. The 25% reduction in CV risk cannot therefore be attributed to statistical chance or to any theoretical mineral oil contribution. Further scrutiny showed that the advantage of IPE over other omega-3 fatty acids might be conferred by the greater stability of this omega-3 fatty acid that exerts its lipid-lowering effects through EPA. Indeed, achieved serum EPA levels directly correlate with CV protection, regardless of their TG-lowering effects; since TG is a mediator of CVD and lower TG levels do not necessarily imply lower CV risk. EPA is believed to mitigate this CVD risk in patients with moderate or high levels of TG. As per REDUCE-IT, IPE resulted in the highest serum EPA levels and was associated with a 25% CV risk reduction [103], compared to lower serum EPA levels in patients administered other forms of omega-3 fatty acids [92].
How is IPE Positioned in the International Dyslipidemia Guidelines?
Year 2021 witnessed the endorsement of IPE by many international medical societies. Table 3 lists the international recommendations endorsing the use of IPE, along the road to its approval and up to currently applicable guidelines.
Which Patients are Candidates for IPE Treatment?
Patients with high TG levels and controlled LDL-cholesterol levels were enrolled in REDUCE-IT and those in the IPE treatment arm showed significantly lower risk for new-onset or recurring CVD.
IPE can be used for primary or secondary prevention purposes. Table 4 lists the risk-enhancing criteria that make patients eligible for IPE treatment, depending on clinical phenotype. Interestingly, there is a robust medical profile that qualifies patients for primary prevention use of IPE.
Patients with dyslipidemia (particularly elevated TG levels), in addition to those with other morbid conditions and with different smoking histories, as shown in Fig. 1, greatly benefit from IPE treatment. While all patients with CV risk factors might benefit from IPE treatment for primary prevention, patients with atherogenic dyslipidemia, i.e., patients with TG levels beyond 2.26 mmol/l (200 mg/dl) and HDL-cholesterol levels below 0.9 mmol/l (35 mg/dl) are likely to derive the greatest CV protection from the addition of IPE to their regular statin-based therapy [52]. Importantly, routine screening for high CV risk and risk enhancers remains suboptimal in clinical practice; however, this inertia invariably leads to significant residual CV risk. In fact, guidelines recommend identification of the different CV risk factors and the stratification of patients to inform best therapeutic decisions. Therefore, every patient fulfilling any of the below CV risk enhancers must be evaluated for additional lipid-lowering therapy in order to target residual CV risk. As a rule of thumb, clinical care starts by aggressively targeting LDL-cholesterol with statins and then adding ezetemibe, PCSK9 inhibitors, or IPE to patients who achieved controlled LDL-cholesterol levels or to those with statin resistance or intolerance. Patients with severe mixed dyslipidemia should invariably be on the highest tolerated statin dose, which should be optimized before starting IPE. Patients with severe hypertriglyceridemia are also at risk of pancreatitis, and these patients can be prescribed IPE on top of statins, and then as needed, fibrates.
Are the Safety Events Reported with IPE Use Clinically Relevant in the Context of CV Risk Reduction?
The net clinical benefit of IPE was sustained in all treated population subgroups and far outweighs potential risks. In fact, while the proportion of any bleeding was higher (P = 0.006) in the IPE group (11.8%) compared to placebo (9.9%)), the rate of hemorrhagic stroke did not reach statistical significance (P = 0.54), while serious bleeding events were more frequent in the IPE group (2.7% [111/4089] versus 2.1% [85/4090] in the placebo group, P = 0.06) . In addition, the frequency of bleeding events in aspirin-treated patients is higher than that reported upon IPE use [113]. More atrial fibrillation/flutter events were recorded in the IPE group (P = 0.002), including serious ones requiring hospitalization longer than 24 h (P = 0.008) [52]. However, major CV events, including stroke, were still reduced in the IPE arm, and some patients already had a history of atrial fibrillation at baseline. Indeed, recurrent atrial fibrillation/flutter was more frequent than de novo atrial fibrillation/flutter and those patients especially experienced marked CV benefits upon IPE use [114]. A subgroup analysis of REDUCE-IT showed that rates of hospitalization for atrial fibrillation were higher in patients with prior atrial fibrillation (12.5% in the IPE versus 6.3% in the placebo group, P = 0.007), compared to patients without history of atrial fibrillation (2.2% versus 1.6%, respectively, P = 0.09) [115].
Overall, fewer CV events occurred in the IPE arm, compared to placebo [113] and, in particular, the risk of an increase in hospitalization due to atrial fibrillation/flutter and the trend toward increased serious bleeding risk with IPE are offset by its CV benefits [114, 115]. In addition, recommendation for IPE use by international scientific and medical associations attests to the fact that its potential benefits greatly outweigh the safety concerns that should, nevertheless, continue to be monitored in clinical practice.
How to Leverage Residual CV Risk Surveillance and Management at the Primary Care Level?
While CVD should be diagnosed and managed at secondary or tertiary care centers, by a multidisciplinary team of experienced cardiologists and other specialties, primary care remains important for the identification of CV risk factors and early signs of CVD in the majority of the population with no prior CV event. In fact, “red flags” can be picked up by any physician, and screening for risk enhancers must be routinely performed in primary care settings. Risk enhancers include family history of atherosclerotic CVD, metabolic syndrome, persistently elevated LDL-cholesterol, low HDL-cholesterol, elevated TG levels, high hsCRP levels, chronic kidney disease, chronic inflammatory diseases, etc. [116]. Patients eligible for lipid-lowering treatment can be evaluated for eligibility for IPE in primary or secondary prevention; hence the need to train primary care physicians and nurses to evaluate patients, according to criteria listed in Table 2. Even patients with varying (elevated or modestly elevated; i.e., TG > 1.69 mmol/l or 150 mg/dl) TG levels can benefit from IPE. From a surveillance standpoint, patients from increased risk groups and patients with modest elevation of TG will be provided with greater CV protection with IPE; which should be prescribed at the primary care level. The following URL (https://www.acc.org/~/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Guidelines/2018/Guidelines-Made-Simple-Tool-2018-Cholesterol.pdf) links to a document that can serve to direct screening and management of CV risk-enhancing factors at primary care settings.
To What Extent Does Health Hygiene (Including Fish-Rich Diet) impact CVD Onset, Progression or Regression in Patients at High CV Risk or with Residual CV Risk?
Unhealthful behaviors predispose individuals to a plethora of health conditions, underscoring the importance of primordial prevention in the general population or primary prevention in the population at risk for certain diseases. In particular, CVD are largely preventable if underlying risk factors are addressed in a timely manner. Regular physical activity, abstinence from smoking, low-fat and low-sugar diets, and moderate alcohol consumption and avoidance of pollutants are of utmost importance in preventing CVD or in mitigating symptoms and delaying complications. It is widely accepted that populations with high fatty fish intake and with elevated serum omega-3 fatty acid levels have traditionally lower CV risk and greater longevity [117, 118]. Despite the epidemiological data offering a glimpse of hope for the prevention of CVD and outcome exacerbations [119, 120], trials exploring omega-3 fatty acid-rich fish oil supplementation have failed to present evidence on improved CV outcomes in patients with CVD [78, 92, 93, 121]. This limited CV benefit of omega-3 fatty acids can be attributed to changes in their physical properties, but also to the fact that patients in these trials all had established CVD. Although nutrition and population-based studies underscore the importance of omega-3 fatty acids, therapeutic levels of serum EPA are difficult to attain from a sporadic omega-3-rich diet; coupled with the impracticality of motivating dietary habit change in populations with specific cultural and socio-economic underpinnings.
In Terms of Health Economics, what are the Benefits of Using IPE to Further Mitigate CV Risk in Statin-Treated Patients?
Some people with limited financial resources might turn to free or cheaper treatment alternatives to avoid paying charges, but this approach might result in higher rather than lower healthcare expenditure [122]. Rosuvastatin alone or with candesartan and hydrochlorothiazide proved a cost-saving prescription in higher income countries, but resulted in higher expenditure on health in developing countries [123]. With particular interest in dyslipidemia management, treatment with PCSK9 inhibitors to decrease levels of LDL-cholesterol was reported in 2016 to be too expensive and unaffordable by health systems [124] and in 2018, the American College of Cardiology guidelines concluded that the price of PCSK9 inhibitors must be reduced by almost 70% to meet cost-effectiveness standards [125]; although a more recent study has proven PCSK9 inhibitors to be cost-effective only at a substantially lower cost and in the very-high-risk population [126]. A very recent study evaluated the cost-effectiveness of adding IPE treatment to statin treatment for CV risk reduction and found that it would be especially cost-effective in the context of secondary prevention of CVD [127]. Importantly, REDUCE-IT shows that CV event frequency decreases upon IPE treatment [80], which inherently improves clinical outcomes and decreases the use of healthcare resources. In the United States, IPE was found to be cost-effective in primary prevention and associated with even better outcomes at lower cost for secondary prevention purposes [128]. The clinical need is undeniable, provided the acquisition cost of IPE fits with the budget of stakeholders involved in medication provision and coverage (government subsidies, third-party payers, out-of-pocket expenditure, etc.). Patients and physicians alike should be made aware that the higher cost of IPE is offset by its effectiveness in preventing CVD, in reducing the recurrence of CV events that require medical care, and in mitigating overall mortality and CV burden.
Conclusion
Elevated TG levels are not only a biomarker of CV risk, but also a mediator of CVD, conferring a significant residual CV risk persisting in patients treated with lipid-lowering agents. Fundamentally, even modestly elevated TG levels are a risk factor that can be very effectively managed with a specific agent (IPE) that has very robust evidence in reducing CV risk. Mixed omega-3 fatty acid formulations have been shown to decrease TG levels, without, however, eradicating the residual CV risk. IPE, through the robust and unequivocally positive REDUCE-IT results, revolutionized the use of pure omega-3 fatty acids in primary and secondary prevention of CVD. IPE showed marked and sustained CV benefits in patients with high TG and low HDL-cholesterol levels, but also in patients presenting with comorbid conditions, known as CV risk enhancers, metabolic syndrome and atherogenic dyslipidemia, which are particularly prevalent in some populations such as in the Middle East. IPE was indeed evaluated in different population subgroups, which are clinically relevant in daily practice; as reflected in the most updated guidelines. IPE seems to exert its multiple CV protective effects through the attenuation of the inflammatory response, the stabilization of the plasma membrane, the regression of atherosclerosis and, ultimately, the reinstatement of normal vascular function. IPE is also generally well tolerated, despite a higher risk of bleeding and atrial fibrillation, which did not compromise the strong clinical net benefit of IPE. Since its approval by the Food and Drug Administration in 2019 and then by the European Medicines Agency in 2021, IPE has shifted the paradigm of hypertriglyceridemia management in clinical practice [129]. The rather broad benefits conferred by IPE make it cost-effective at a societal level, which should prompt its rapid adoption as a pillar of treatment for both dyslipidemia and CV risk.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Hermans MP, Fruchart JC. Reducing vascular events risk in patients with dyslipidaemia: an update for clinicians. Ther Adv Chronic Dis. 2011;2(5):307–23.
Dhindsa DS, Sandesara PB, Shapiro MD, Wong ND. The evolving understanding and approach to residual cardiovascular risk management. Front Cardiovasc Med. 2020;7:88.
Vanuzzo D. The epidemiological concept of residual risk. Intern Emerg Med. 2011;6(Suppl 1):45–51.
Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–74.
Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361(22):2113–22.
Burger AL, Pogran E, Muthspiel M, et al. New treatment targets and innovative lipid-lowering therapies in very-high-risk patients with cardiovascular disease. Biomedicines. 2022;10(5) https://doi.org/10.3390/biomedicines10050970.
Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–72.
Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97.
Guo Y, Cui L, Ye P, et al. Change of kidney function is associated with all-cause mortality and cardiovascular diseases: results from the Kailuan study. J Am Heart Assoc. 2018;7(21):e010596.
The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. Jama. 1984;251(3):365–74.
Yao YS, Li TD, Zeng ZH. Mechanisms underlying direct actions of hyperlipidemia on myocardium: an updated review. Lipids Health Dis. 2020;19(1):23.
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40(1):195–211.
Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–57.
Ballantyne CM. Low-density lipoproteins and risk for coronary artery disease. Am J Cardiol. 1998;82(9a):3q–12q.
Brown BG. Maximizing coronary disease risk reduction using nicotinic acid combined with LDL-lowering therapy. Eur Heart J Suppl. 2005;7(suppl_F):F34–40.
Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. Jama. 1998;279(20):1615–22.
Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39.
Navar AM, Pagidipati N, Mulder H, et al. (2019) Triglycerides as a risk factor for coronary heart disease: what measure and what cutoff. in 68th Annual Scientific Session of the American College of Cardiology. New Orleans, LA
Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New Eng J Med. 2008;359(21):2195–207.
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–9.
Scandinavian Simvastatin Survival Study, G. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–9.
Shepherd J, Cobbe SM, Ford I, et al. Prevention of Coronary Heart Disease with Pravastatin in Men with Hypercholesterolemia. New Eng J Med. 1995;333(20):1301–8.
Miller M, Cannon CP, Murphy SA, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–30.
Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267–75.
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–8.
Graham I, Cooney MT. Risks in estimating risk. Eur Heart J. 2013:35. https://doi.org/10.1093/eurheartj/eht286.
Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33(13):1635–701.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Blackburn DF, Lamb DA, Eurich DT, et al. Atenolol as initial antihypertensive therapy: an observational study comparing first-line agents. J Hypertens. 2007;25(7):1499–505.
Jardine MJ, Ninomiya T, Perkovic V, et al. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010;56(12):956–65.
Ruilope LM, Salvetti A, Jamerson K, et al. Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol. 2001;12(2):218–25.
Zanchetti A, Hansson L, Dahlöf B, et al. Benefit and harm of low-dose aspirin in well-treated hypertensives at different baseline cardiovascular risk. J Hypertens. 2002;20(11):2301–7.
Turnbull F, Neal B, Algert C, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165(12):1410–9.
Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–16.
Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96.
Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29(6):1220–6.
Na E, Cho S, Kim DJ, Choi J, Han E. Time-varying and dose-dependent effect of long-term statin use on risk of type 2 diabetes: a retrospective cohort study. Cardiovascular Diabetology. 2020;19(1):67.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am College Cardiol. 2019;73(24):3168–209.
Mach F, Ray KK, Wiklund O, et al. Adverse effects of statin therapy: perception vs. the evidence - focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur Heart J. 2018;39(27):2526–39.
Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301–10.
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–504.
Shapiro MD, Bhatt DL. "Cholesterol-Years" for ASCVD Risk Prediction and Treatment. J Am Coll Cardiol. 2020;76(13):1517–20.
Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2015;36(13):774–6.
Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. Jama. 2019;321(4):364–73.
Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet Need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330–43.
Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112(1):25–31.
Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–8.
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65.
Bhatt DL, Topol EJ. Need to Test the Arterial Inflammation Hypothesis. Circulation. 2002;106(1):136–40.
Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. Jama. 2001;286(1):64–70.
Muhammad K, Morledge T, Sachar R, et al. Treatment with ω-3 fatty acids reduces serum C-reactive protein concentration. Clinical Lipidology. 2011;6:723–9.
Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. New Eng J Med. 2018;380(1):11–22.
Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity C-reactive protein after an initially elevated concentration: the JUPITER Study. Clin Chem. 2009;55(2):305–12.
Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–8.
Watanabe T, Ando K, Daidoji H, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017;70(6):537–44.
Budoff MJ, Muhlestein JB, Bhatt DL, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: a prospective, placebo-controlled randomized trial (EVAPORATE): interim results. Cardiovasc Res. 2020;117(4):1070–7.
Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41(40):3925–32.
Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31(3):234–45.
Patel PN, Patel SM, Bhatt DL. Cardiovascular risk reduction with icosapent ethyl. Curr Opin Cardiol. 2019;34(6):721–7.
Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–61.
Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. New Eng J Med. 2010;362(17):1563–74.
Pradhan AD, Paynter NP, Everett BM, et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J. 2018;206:80–93.
Das Pradhan A, Glynn RJ, Fruchart JC, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med. 2022;387(21):1923–34.
Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. New Eng J Med. 2011. 365(24):2255-2267. https://doi.org/10.1056/NEJMoa1107579.
The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. New Eng J Med. 2014. 371(3):203-212. https://doi.org/10.1056/NEJMoa1300955.
Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999. 354(9177):447-55.
Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy - Statin and Eicosapentaenoic Acid (RESPECT-EPA). (UMIN Clinical Trials Registry Number: UMIN000012069). 2022; Available from: https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014051.
Preston Mason R. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atherosclerosis Rep. 2019;21(1):2.
Toth PP, Chapman MJ, Parhofer KG, Nelson JR. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. Am Heart J Plus: Cardiol Res Pract. 2022;17:100148.
Bavry AA and Bhatt DL. Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid - RESPECT-EPA. In American Heart Association Scientific Sessions. 2022. Chicago, IL.
Bhatt DL, Steg PG, Brinton EA, et al. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin Cardiol. 2017;40(3):138–48.
Bosch J, Gerstein HC, Dagenais GR, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–18.
Roncaglioni MC, Tombesi M, Avanzini F, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–8.
Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152–9.
Bowman L, Mafham M, Wallendszus K, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540–50.
Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–43.
Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk: a secondary analysis of the STRENGTH Trial. JAMA Cardiol. 2021;6(8):1–8.
Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225–33.
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–9.
Bhatt DL, Steg PG, Miller M, et al. Reduction in first and total ischemic events with icosapent ethyl across baseline triglyceride tertiles. J Am College Cardiol. 2019;74(8):1159–61.
Bhatt DL, Brinton EA, Miller M, Steg G, Jacobson TA, Ketchum SB, Juliano RA, Jiao L, Doyle RT Jr, Granowitz C, Ganda O, Welty FK, Busch RS, Goldberg AC, Herrington DM, Budoff M, Tardif JC, Ballantyne CM. Icosapent Ethyl Provides Consistent Cardiovascular Benefit in Patients with Diabetes in REDUCE-IT. On behalf of the REDUCE-IT investigators. https://www.acc.org/education-and-meetings/image-and-slide-gallery/media-detail?id=EF9739685864453B84D5E26AC534A152
Peterson BE, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Juliano RA, Jiao L, Doyle RT Jr, Granowitz C, Gibson CM, Pinto D, Giugliano RP, Budoff MJ, Tardif JC, Verma S, Ballantyne CM; REDUCE-IT Investigators. Reduction in revascularization with icosapent ethyl: insights from REDUCE-IT revascularization analyses. Circulation. 2021;143(1):33–44. https://doi.org/10.1161/CIRCULATIONAHA.120.050276.
Peterson B, Bhatt D, Steg P, Miller M, Brinton E, Ketchum S, Juliano R, Jiao L, Doyle R, Granowitz C, Pinto D, Giugliano R, Budoff M, Tardif J‐C, Verma S, Ballantyne C. Treatment with Icosapent Ethyl to Reduce Ischemic Events in Patients with Prior Percutaneous Cornoary Intervention: Insights From REDUCE-IT PCI. J Am Coll Cardiol. 2020;76(17):1–2. https://doi.org/10.1016/j.jacc.2020.09.018.
Verma S, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Dhingra NK, Ketchum SB, Juliano RA, Jiao L, Doyle RT Jr, Granowitz C, Gibson CM, Pinto D, Giugliano RP, Budoff MJ, Mason RP, Tardif JC, Ballantyne CM; REDUCE-IT Investigators. Icosapent ethyl reduces ischemic events in patients with a history of previous coronary artery bypass grafting: REDUCE-IT CABG. Circulation. 2021;144(23):1845–1855. https://doi.org/10.1161/CIRCULATIONAHA.121.056290.
Wang X, Bhatt DL, Miller M, Steg Ph.G, Brinton EA, Jacobson TA, Ketchum SB, Juliano RA, Jiao L, Doyle RT, Granowitz Jr. C, Copland C, Tardif J-C, Ballantyne CM, Budoff MJ, Mason RP, Boden WE, on Behalf of the REDUCE-IT Investigators, “Icosapent Ethyl Reduces Ischemic Events in Patients with High Triglycerides and Low High-Density Lipoprotein Cholesterol Levels: REDUCE-IT High TG/Low HDL-C Analyses”, and it is available at: https://www.acc.org/education-and-meetings/image-and-slide-gallery/media-detail?id=3f7acc9e9c254784924249b5e2fd4
2021 ISC presentation (virtual), Bhatt DL, Steg Ph. G, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Juliano RA, Jiao L, Doyle RT, Granowitz Jr. C, Tardif J-C, Gregson J, Gibson CM, Leary MC, Ballantyne CM, on Behalf of the REDUCE-IT Investigators, “Reductions in Ischemic Stroke with Icosapent Ethyl: Insights From REDUCE-IT”, available online at ACC.org: https://www.acc.org/education-and-meetings/image-and-slide-gallery/media-detail?id=c1fd107853f84abca5e552511ae756e7
Bhatt DL, Brinton EA, Miller M, Steg Ph. G, Ketchum SB, Juliano RA, Jiao L, Doyle RT, Granowitz Jr. C, Tardif J-C, Ballantyne CM, on Behalf of the REDUCE-IT Investigators, ADA 2021 (virtual), “Substantial Cardiovascular Risk Reduction with Icosapent Ethyl Regardless of BMI or Diabetes Status: REDUCE-IT BMI”. Restricted (login) access online at ADA: https://professional.diabetes.org/webcast/substantial-cardiovascular-risk-reduction-icosapent-ethyl-regardless-diabetes-status-or-bmie0d
Miller M, Bhatt DL, Steg PG, et al. Potential effects of icosapent ethyl on cardiovascular outcomes in cigarette smokers: REDUCE-IT Smoking. Eur Heart J Cardiovasc Pharmacother. 2022; https://doi.org/10.1093/ehjcvp/pvac045.
Wang X, Verma S, Mason RP, Bhatt DL. The Road to Approval: a Perspective on the Role of Icosapent Ethyl in Cardiovascular Risk Reduction. Curr Diab Rep. 2020;20(11):65.
Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arteriosclerosis, Thrombosis Vas Biol. 2020;40(5):1135–47.
Alfaddagh A, Martin SS, Leucker TM, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol. 2020;4:100130.
Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH Randomized Clinical Trial. JAMA. 2020;324(22):2268–80.
Kalstad AA, Myhre PL, Laake K, et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation. 2021;143(6):528–39.
Kastelein JJP, Stroes ESG. FISHing for the miracle of eicosapentaenoic acid. N Engl J Med. 2019;380(1):89–90.
Olshansky B, Chung MK, Budoff MJ, et al. Mineral oil: safety and use as placebo in REDUCE-IT and other clinical studies. Eur Heart J Suppl. 2020;22(Supplement_J):J34–48.
Ridker PM, Rifai N, MacFadyen J, et al. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin-1β, interleukin-6, C-reactive protein, oxidized low-density lipoprotein cholesterol, homocysteine, lipoprotein(a), and lipoprotein-associated phospholipase A2: a REDUCE-IT biomarker substudy. Circulation. 2022;146(5):372–9.
Singh N, Bhatt DL, Miller M, et al. Consistency of benefit of icosapent ethyl by background statin type in REDUCE-IT. J Am College Cardiol. 2022;79(2):220–2.
Sherratt SC, Libby P, Bhatt DL, Mason P. Abstract 13685: Eicosapentaenoic Acid (EPA) Inhibits Low-Density Lipoprotein (LDL) Oxidation Compared to Docosahexaenoic Acid (DHA) and Mineral Oil in vitro. Circulation. 2022;146(Suppl_1):A13685.
Sherratt SCR, Libby P, Bhatt DL, Mason RP. Comparative effects of mineral oil, corn oil, eicosapentaenoic acid, and docosahexaenoic acid in an in vitro atherosclerosis model. J Am Heart Assoc. 2023;12(7):e029109.
Puri R, Libby P, Nissen SE, et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging. 2014;15(4):380–8.
Nissen SE, Nicholls SJ. Results of the GLAGOV trial. Cleve Clin J Med. 2017;84(12 Suppl 4):e1–5.
Tokgozoglu L, Catapano AL. Can EPA evaporate plaques? Eur Heart J. 2020;41(40):3933–5.
Bhatt D. EPA levels and cardiovascular outcomes in the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. in ACC.20/Wcc. 2020. Chicago.
Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364–467.
Virani SS, Morris PB, Agarwala A, et al. 2021 ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;78(9):960–93.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227–337.
COUNCIL, S.N.D.C.S.a.t.S.H., Saudi Diabetes Clinical Practice Guidelines (SDCPG). 2021.
American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S111–S134. https://doi.org/10.2337/dc20-S010.
Arnold SV, Bhatt DL, Barsness GW, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2020;141(19):e779–806.
Gladstone DJ, Lindsay MP, Douketis J, et al. Canadian Stroke Best Practice Recommendations: Secondary Prevention of Stroke Update. Can J Neurol Sci. 2020;2021:1–23.
2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019. 290: p. 140-205. https://doi.org/10.1016/j.atherosclerosis.2019.08.014.
Orringer CE, Jacobson TA, Maki KC. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J Clin Lipidol. 2019;13(6):860–72.
Parhofer KG, Chapman MJ, Nordestgaard BG. Efficacy and safety of icosapent ethyl in hypertriglyceridaemia: a recap. Eur Heart J Suppl. 2020;22(Supplement_J):J21–33.
Olshansky B, Bhatt D, Miller M, et al. Cardiovascular benefits outweigh risks in patients with atrial fibrillation in REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial). Eur Heart J. 2021;42(Supplement_1). https://doi.org/10.1093/eurheartj/ehab724.2568.
Olshansky B, Bhatt DL, Miller M, et al. Cardiovascular benefits of icosapent ethyl in patients with and without atrial fibrillation in REDUCE IT. J Am Heart Assoc. 2023;12(5):e026756.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am College Cardiol. 2019;73(24):e285–350.
Harris WS, Tintle NL, Imamura F, et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nature Commun. 2021;12(1):2329.
Umesawa M, Yamagishi K, Iso H. Intake of fish and long-chain n-3 polyunsaturated fatty acids and risk of diseases in a Japanese population: a narrative review. Eur J Clin Nutr. 2021;75(6):902–20.
Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312(19):1205–9.
Zheng J, Huang T, Yu Y, et al. Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Public Health Nutrition. 2011;15(4):725–37.
Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460–2.
Gemmill MC, Thomson S, Mossialos E. What impact do prescription drug charges have on efficiency and equity? Evidence from high-income countries. Int J Equity Health. 2008;7(1):12.
Lamy A, Lonn E, Tong W, et al. The cost implication of primary prevention in the HOPE 3 trial. Eur Heart J Qual Care Clin Outcomes. 2019;5(3):266–71.
Arbel R, Hammerman A, Triki N, Greenberg D. PCSK9 inhibitors may improve cardiovascular outcomes-Can we afford them? Int J Cardiol. 2016;220:242–5.
Arrieta A, Hong JC, Khera R, et al. Updated cost-effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER Trial. JAMA Cardiol. 2017;2(12):1369–74.
Robinson JG. Lipid management beyond the guidelines. Prog Cardiovasc Dis. 2019;62(5):384–9.
Bazarbashi N, Miller M. Icosapent ethyl: niche drug or for the masses? Curr Cardiol Rep. 2020;22(10):104.
Weintraub WS, Bhatt D, Zhang Z, et al. Cost-effectiveness of icosapent ethyl in us reduce-it patients. J Am College Cardiol. 2020;75(11_Supplement_1):1914.
Ademi Z, Ofori-Asenso R, Zomer E, Owen A, Liew D. The cost-effectiveness of icosapent ethyl in combination with statin therapy compared with statin alone for cardiovascular risk reduction. Eur J Prev Cardiol. 2021;28(8):897–904.
Acknowledgements
The authors would like to acknowledge KBP-Biomak, a contract research organization, for medical writing and editorial support funded by Biologix.
Funding
This work was supported by an unrestricted grant from Biologix. Biologix provided financial and technical support for the expert panel meeting, which laid down the foundation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated equally in this study, acquisition of information, drafting, and reviewing the manuscript. They all revised and critically reviewed all versions of the manuscript and they approve it in its current form.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
Author Hani Sabbour is a speaker at Biologix events. All his honoraria were donated to patient-supporting charities in the UAE.
Author Deepak Bhatt discloses the following relationships – Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Angiowave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures, Hims; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, http://ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither I nor Brigham and Women’s Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda.
Author Layal Bennani is an employee of Biologix.
Yaser Elhenawi, Asma Aljaberi, Tarek Fiad, Khwaja Hasan, Shahrukh Hashmani, Rabih A. Hijazi, Zafar Khan and Ronney Shantouf declare no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Statin-treated patients with hypertriglyceridemia remain at residual CV risk.
• Omega-3 polyunsaturated fatty acids especially Icosapentaenoic acid mitigate the residual CV risks in statin-treated patients with persistently elevated triglyceride levels.
• Icosapent ethyl (IPE) exerts the most robust cardioprotective effect among omega-3 polyunsaturated fatty acids and reduces CV events by 25%.
• 20 international medical societies endorse IPE use as an add-on treatment to statin in patients with dyslipidemia at high CV risk.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sabbour, H., Bhatt, D.L., Elhenawi, Y. et al. A Practical Approach to the Management of Residual Cardiovascular Risk: United Arab Emirates Expert Consensus Panel on the Evidence for Icosapent Ethyl and Omega-3 Fatty Acids. Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-023-07519-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07519-z