Abstract
Purpose
Patients hospitalized with COVID-19 may develop a hyperinflammatory, dysregulated cytokine “storm” that rapidly progresses to acute respiratory distress syndrome, multiple organ dysfunction, and even death. Remote ischaemic conditioning (RIC) has elicited anti-inflammatory and cytoprotective benefits by reducing cytokines following sepsis in animal studies. Therefore, we investigated whether RIC would mitigate the inflammatory cytokine cascade induced by COVID-19.
Methods
We conducted a prospective, multicentre, randomized, sham-controlled, single-blind trial in Brazil and South Africa. Non-critically ill adult patients with COVID-19 pneumonia were randomly allocated (1:1) to receive either RIC (intermittent ischaemia/reperfusion applied through four 5-min cycles of inflation (20 mmHg above systolic blood pressure) and deflation of an automated blood-pressure cuff) or sham for approximately 15 days. Serum was collected following RIC/sham administration and analyzed for inflammatory cytokines using flow cytometry. The endpoint was the change in serum cytokine concentrations. Participants were followed for 30 days.
Results
Eighty randomized participants (40 RIC and 40 sham) completed the trial. Baseline characteristics according to trial intervention were overall balanced. Despite downward trajectories of all cytokines across hospitalization, we observed no substantial changes in cytokine concentrations after successive days of RIC. Time to clinical improvement was similar in both groups (HR 1.66; 95% CI, 0.938–2.948, p 0.08). Overall RIC did not demonstrate a significant impact on the composite outcome of all-cause death or clinical deterioration (HR 1.19; 95% CI, 0.616–2.295, p = 0.61).
Conclusion
RIC did not reduce the hypercytokinaemia induced by COVID-19 or prevent clinical deterioration to critical care.
Trial Registration
ClinicalTrials.gov Identifier: NCT04699227.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus-19 disease (COVID-19) emerged in late 2019 and has since placed an enormous strain on healthcare resources, particularly in low- to middle-income countries (LMIC). There are currently well over 500 million cases documented worldwide, with approximately 6 million deaths reported globally at the time of writing [1]. About 15% of infected adults are hospitalized with severe COVID-19 pneumonia, and a subset rapidly progresses to acute respiratory distress syndrome (ARDS), multiple organ dysfunction syndrome (MODS), and even death [2, 3]. Early studies have correlated the presence of a cytokine “storm” in patients with COVID-19 and severe outcomes [4]. Interleukin (IL)-6 and tumor necrosis factor (TNF)-α have been considered by many as major cytokine culprits in the pathogenesis of this COVID-19-induced hyper-inflammation [5, 6]. Cytokine “storm” syndrome is a complex release of multiple cytokines in response to pathogenic material, such as SARS-CoV-2 [2]. Considering the high mortality and elevated pro-inflammatory cascade in those that deteriorate, suppressing this cytokine “storm” phenomenon may dampen immune hyperactivity and serve as a complementary therapeutic strategy as we advance our strategies against COVID-19 [7,8,9]. Previous trials have demonstrated a close link between COVID-19 disease severity and elevated IL-6 levels; however, most IL-6 neutralizing agents, such as tocilizumab, have shown modest benefit in patients hospitalized with COVID-19 [10, 11]. Dexamethasone, which has broad immunosuppressive roles via diverse mechanisms of action, has been shown to reduce lung injury induced by a cytokine storm [12]. However, not all cytokines are pro-inflammatory. Extensive non-tailored immunosuppression may be considered a double-edged sword especially if the delicate cytoprotective balance is compromised. For example, interferon (IFN)-γ is an immunomodulatory agent stimulated during infection and essential for downregulating viral replication. Described as an integral part of the body’s innate antiviral defence system, over-suppression may be harmful [13, 14]. Identifying or repurposing therapies which target the COVID-19-induced cytokine storm while preserving the cytoprotective cascade is therefore essential in halting or reducing the severity of damage in patients with COVID-19 [2].
Several animal models have suggested that the novel, noninvasive, and highly feasible intervention known as remote ischaemic conditioning (RIC) can suppress inflammatory cytokines and elicit cytoprotective and anti-inflammatory benefits in sepsis [15,16,17,18]. Early repetitive or chronic RIC administration has shown benefits in reducing levels of inflammatory cytokines (such as TNF-α, IL-1β, and IL-6) following lipopolysaccharide-induced sepsis and has been associated with a reduction in mortality in mice [17]. In recent years, RIC has grown from being an innovative strategy for cardiovascular protection [19,20,21]. This emerging field of RIC has been thought to protect organs (e.g., brain, heart, lungs, and kidneys) against the harmful effect of ischemic/reperfusion injury through activation of cell survival pathways and modulation of inflammatory responses that improve mitochondrial function [22,23,24,25]. RIC is a safe treatment modality whereby a blood-pressure cuff is applied to the upper arm for repeated cycles of supra-systolic inflation and deflation (typically four 5-min cycles). This process of short cycles of nonlethal ischaemia of the upper limb, followed by reperfusion activates neurohormonal pro-survival mechanisms in the body to protect vital organs at remotely injured sites [16, 18, 26, 27]. Given the pro-inflammatory cytokine cascade elicited by COVID-19, we hypothesized that the therapeutic efficacy of RIC on cytokines induced in sepsis models might be translated to the hypercytokinaemia array seen in hospitalized patients with COVID-19.
Following the RECOVERY trial published in the latter half of 2020, dexamethasone, after demonstrating a significant mortality reduction in hypoxic hospitalized patients with COVID-19, has been rolled out in almost all centres worldwide [28]. It is possible that the immunomodulatory and cytoprotective effects stimulated by RIC, as additional therapy in our anti-COVID-19 artillery, could provide further benefit. However, the ability of RIC to modulate levels of inflammatory cytokines in hospitalized patients with COVID-19 remains to be established [18]. Here, we report the results of a preliminary proof-of-concept study designed to address this knowledge gap on the potential impact of RIC in patients with COVID-19.
Methods
Trial Design
We conducted an investigator-initiated, multicentre, international, single-blind, phase 3, parallel, randomized, sham-controlled trial to evaluate the effects of RIC on inflammatory cytokines in adults (aged ≥ 18 years) admitted to hospital with COVID-19 pneumonia. The trial was implemented in two low- to middle-income countries, Hospital Estadual de Sumaré in Campinas, Brazil, and Groote Schuur Hospital in Cape Town, South Africa (SA). Ethical approvals were obtained from regional health review boards at each participating hospital (State University of Campinas, Brazil: CAAE 33,709,320.4.0000.5404; and Human Research Ethics Committee, University of Cape Town, South Africa: HREC 407/2020) and were conducted in accordance with the principles of good clinical practice and accordance with the Declaration of Helsinki and its later amendments. “RIC in COVID-19” trial protocol was registered at ClinicalTrials.gov (identifier: NCT04699227) before the first randomization, and the study has been conducted and reported according to the CONSORT statement. All participants provided written informed consent before randomization. Details of the trial rationale and design have been previously published [29], and a copy of the protocol is available.
Study Population
“RIC in COVID-19” included men and nonpregnant female patients with confirmed RT-PCR positive for SARS-CoV-2 at both recruiting centres. In addition, patients were included if they had a confirmed diagnosis of COVID-19 pneumonia on chest imaging and did not require critical care support, i.e., mechanical ventilation, vasopressors, or renal replacement therapy. Noninvasive respiratory support was defined as those requiring supplemental oxygen when delivered by nasal cannula, face mask, or high-flow nasal cannula respiratory support. Escalation of respiratory support was defined as the transition from noninvasive respiratory support to ventilation delivered by endotracheal or tracheostomy tube. Key exclusion criteria included contraindications to the usage of a brachial blood pressure cuff on either arm; intercurrent disease with a life expectancy of less than 24 h; recovery post-cardiac arrest; pregnant or breastfeeding women; bleeding disorders or platelet count below 50 × 109/L; severe renal impairment (estimated glomerular filtration rate < 30 mL/min per 1.73 m2) or receipt of haemodialysis or peritoneal dialysis; chronic liver disease and/or ALT and AST ≥ 5 times the standard upper reference limit; significant immunodeficiency states: HIV/AIDS not on antiretroviral agents, solid organ transplants, and bone marrow transplants; chronic use of immunomodulating therapy such as TNF-α or chronic corticosteroids with prednisone-equivalent dose ≥ 20 mg/day; active underlying malignancy; symptomatic chronic obstructive pulmonary disease; baseline stage C chronic heart failure; and enrolment into any other investigational treatment study for COVID-19 in the 30 days before screening.
Study Enrolment and Randomization
Eligible patients were enrolled within 24 h of hospital admission for acute COVID-19 pneumonia. All patients provided written informed consent. Participants were randomly assigned in a 1:1 ratio to receive either remote ischaemic conditioning or sham intervention. All participants received standard medical therapy according to national or local guidelines. Randomization was stratified by country, using a random permuted block size randomization sequence prepared by an independent statistician, and performed via a secure web-based clinical trial support system, i.e., REDCap [30], that was accessible 24 h a day. Randomization was performed by a designated study team member who was unblinded to the treatment allocation. Study participants, treating physicians, and study team members collecting data and assessing outcomes were blinded to treatment allocations.
Trial Intervention
Automated preprogrammed pneumatic sphygmomanometer devices, sponsored by the University College London (UCL) and manufactured by Seagull Aps in Denmark, were used to deliver either the RIC or sham protocol throughout the trial. The RIC protocol comprised of applying a RIC device at enrolment on the upper arm to automatically deliver 4 cycles of 5-min sustained high-pressure cuff inflation (20 mmHg above each participant’s systolic blood pressure) alternating with 5-min sustained cuff deflation (0 mmHg), such that the total RIC protocol took 40 min. The sham protocol comprised the application of a visually identical pneumatic cuff on the upper arm, which automatically delivered sustained low-pressure cuff inflations (20 mmHg) and deflations of a similar frequency and duration as the RIC device. Participants received either RIC or sham on day 0 and were repeated daily for 15 days, or until clinical deterioration or discharge. An unblinded study team investigator applied the RIC and sham devices. Trained study investigators, independent of the treating physicians, assessed device-related adverse events and each participant’s clinical conditioning daily using the World Health Organization (WHO) ten-point Clinical Progression Scale (CPS) [31]. Safety assessments and clinical data were recorded on electronic case report forms that were validated by the trial’s quality control officer.
Cytokine Sampling
Serum samples were prospectively collected from participants at baseline (before trial intervention and within 72 h of hospitalization) and every alternate day following the RIC/sham protocol, where possible, for the analysis of inflammatory cytokines. Samples were stored at − 80 °C and later thawed for batch cytokine analysis. We conducted a multiplex screen for 13 cytokines (IL-1β, IL-6, TNF-α, IP-10, IFN-λ1, IL-8, IL-12, IFN-α2, IFN-λ2/3, GM-CSF, IFN-β, IL-10, and IFN-γ) in a total of 80 COVID-19 participants, using the commercially available LEGENDplex™ Multi-Analyte Flow Assay kit according to the manufacturer’s protocol. Samples from days 0, 2, and 4 were selected as the most common days of sampling across the cohort and were analyzed in duplicate using flow cytometry (BD LSRII Fortessa; BD Biosciences, Franklin Lakes, NJ, USA) to detect cytokine levels. None of the study interventions or procedures delayed or affected the patient’s clinical management of COVID-19 pneumonia at each site.
Study Endpoints
The primary endpoint of this trial was the median change of serum cytokines from admission (day 0) to the fourth day after inclusion. The prespecified secondary endpoints, analyzed in the intention-to-treat population, included (1) time to clinical deterioration (defined as time from randomization to mortality or a two-point reduction of the WHO Clinical Progression Scale, whichever came first) [31], (2) serum IL-6 ≥ 80 pg/mL as a biomarker for severe clinical outcomes in COVID-19 infection, and (3) cytokine score measured by longitudinal mixed-effects modelling. Safety endpoints included device-related adverse events, serious adverse events, and premature discontinuation of the trial intervention.
Study Oversight
This trial was an academic research collaboration between the executive trial steering committee and investigators from UCL, UCT, and Unicamp. The academic research organization at each site coordinated data management. Statistical analyses were performed by the trial statistician using an independent copy of the complete raw dataset. The first version of the manuscript was drafted by the academic authors who take responsibility for the completeness and accuracy of the data and who made the decision to submit the manuscript for publication.
Statistical Methods
Since there is no available data on the effect of RIC on the inflammatory cascade in patients with COVID-19, the sample size was therefore established empirically on 80 participants with a conservative expectation of a small, standardized effect size. The primary endpoint was compared between the RIC and sham groups across all 80 participants and between participants that had or had not deteriorated. The cytokine concentrations were analyzed separately in a longitudinal framework to determine the effect of the randomized treatment at baseline for each cytokine over time. The profile of cytokine concentrations over time exhibited both within-participant variabilities, resulting from repeated cytokine measurements over time for a single participant, and between-participant variabilities due to biological differences between participants included in the study. Therefore, to account for both within-participant and between-participant variabilities, linear mixed-effects models with discrete-time were employed to compare the effect of RIC versus sham on the cytokine concentrations over time. To address the primary aim of the study, the primary covariate, considered to be associated with cytokine concentrations, was the randomized baseline treatment of RIC or sham included as an interaction effect with discrete-time. To resolve between-participant variabilities for each cytokine response, random effects were imposed on the final model’s intercept and slope for discrete-time. The significance of the fixed effects in the model was assessed using conditional t-tests. To determine the random effects, all models were initially fit using maximum likelihood estimation, and model fit was evaluated using the Bayesian Information Criterion (BIC). The final model parameters were estimated using restricted maximum likelihood to obtain unbiased estimates of the variance components of the final model chosen by its BIC. The secondary time-to-event outcome was compared between the RIC and sham groups using Cox regression modelling stratified by the two components of the study on an intention-to-treat basis and presented with Kaplan–Meier curves to assess the total number of outcomes experienced by both groups up to 30 days post-hospital discharge. Further subgroup analysis of the primary outcome of death or deterioration was performed using a multivariate Cox proportional hazard model with a treatment-risk factor interaction included individually for each subgroup. The statistical analysis was performed in R, version 4.1.0.
Data Availability
Data will be disclosed on request and approval of the proposed use by the trial steering committee. In addition, de-identified individual participant data will be made available, as well as data dictionaries and the study protocol. Data will be available for 5 years after the main study publication.
Results
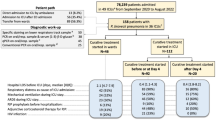
Between January 15th, 2021, and August 31st, 2021, a total of 80 participants at 2 sites on either side of the Atlantic Ocean (40 in Brazil and 40 in South Africa) were randomly allocated. No participants withdrew from the study, and all participants were followed up for 30 days post-hospital discharge or death until a common study end date of October 31st, 2021. Forty participants were assigned and received RIC in the RIC treatment group, and 40 participants were assigned and received the sham intervention in the control treatment group. All 80 participants were included in the intention-to-treat analysis (Fig. 1). The RIC and sham interventions were completed according to the study protocol in all participants across both groups, and the results were included in the per-protocol analysis. No device-related adverse events from the RIC group were observed. Prior to randomization, 64 (80%) of 80 participants received systemic corticosteroids, including 34 (85%) of 40 participants who received RIC and 30 (75%) of 40 participants who received sham. Baseline characteristics according to the treatment groups were balanced and are summarized in Tables 1 and 2. The median age of the participants was 56 (IQR 50–67) years. A total of 48.8% of the participants were female, with a majority (68.8%) presenting with moderate COVID-19 disease (as defined by the WHO CPS severity scale) [31]. Obesity (63.7%) and hypertension (52.5%) were the most observed risk factors, with 32.5% having type 2 diabetes. Only 7.5% of the study population were HIV positive on antiretroviral therapy. The most common symptoms at presentation included cough (77.5%) and shortness of breath (87.5%), with the estimated average onset of symptoms beginning 9 (IQR 6–11) days before admission. All participants were admitted for hypoxemia requiring noninvasive respiratory support with a mean oxygen saturation of 93% (89–96%). Transition to invasive respiratory support occurred in 40% (32/80) of the trial population, with no significant differences between both treatment groups (p = 0.65). At discharge, 49 (61.25%) of 80 participants demonstrated clinical improvement, a median of 5 days shorter in the RIC group (Fig. 2a) (hazard ratio, 1.66; 95% CI, 0.938 to 2.948; p = 0.08). The composite outcome of clinical deterioration or death occurred in 37 (46.3%) participants. Overall, there were no significant differences between the study groups and the probability of death (Fig. 2b) (hazard ratio, 1.35; 95% CI, 0.650 to 2.776; p = 0.41). At follow-up, compared to sham, RIC had no significant impact on the composite outcome of all-cause death or clinical deterioration (hazard ratio, 1.19; 95% CI, 0.616 to 2.295; p = 0.61).
Cytokine Analysis
We conducted a multiplex screen for 13 cytokines in all 80 participants. We monitored the effects of RIC and sham on the selected inflammatory cascade from baseline to the second and fourth days of hospitalization, presented in Table 3. Variances of each cytokine undergoing RIC and sham intervention were further assessed between those that had deteriorated requiring critical care support and those that did not and presented in Tables 4 and 5. By utilizing linear mixed-effects modelling with discrete-time to adjust for both within-participant and between-participant cytokine variabilities at baseline, we were able to account for and further compare the effects of RIC and sham on all 13 immune cytokine profiles. As shown, the proportional and absolute between-group differences in cytokine concentrations varied considerably at baseline and across hospitalization. Despite the downward trajectories of all cytokines across hospitalization, there were no significant differences between the median change in cytokine concentrations from baseline in those who received the RIC intervention compared to sham with respect to the primary endpoint on day 2 or day 4. IL-8 showed a greater reduction from baseline to day 4 in the RIC group; however, it demonstrated a significant difference in baseline concentrations between both groups. Contrastingly, TNF-α showed a relative increase from baseline to day 4 in both groups. At baseline, 43.62% (35/80) of the cohort presented with IL-6 values > 80 pg/mL, of which 54.3% deteriorated or died. Although not significant, IL-6 concentrations were observed to be higher across both arms in the subgroup of participants that deteriorated compared to those that did not, with lower IL-6 values observed on day 4 after receiving RIC in the group of participants that did not require critical support (Table 5). Finally, IL-10 and IL-12 showed similar variations across both groups, with baseline values higher in the sham group, subsequently affecting the median change in cytokine concentrations during analysis (Fig. 3).
Discussion
In this first study of its kind designed to assess the impact of RIC on inflammatory cytokines in participants admitted to hospital with COVID-19, we were unable to detect a significant reduction by RIC in levels of pro-inflammatory cytokines (including IL-1β, IL-6, and TNF-α). Furthermore, RIC did not prevent clinical deterioration or reduce mortality at 30 days.
The cytokine storm induced by COVID-19 served as a novel target for RIC, which has been found to influence multiple pro-survival pathways in a number of clinical settings [16, 18, 26, 27]. In order to test the potential impact of RIC in the setting of COVID-19, we selected a panel of pro-inflammatory cytokines that included IL-6, TNF-α, IL-1β, and IFN-γ. Although RIC has been shown to lower levels of IL-6, TNF-α, and IL-1β [15, 17] and improve survival in animal models of sepsis, whether this concept inspired by animal studies has an impact on inflammatory cytokines stimulated by COVID-19 has not been previously explored. Almost half of our cohort presented with IL-6 concentrations > 80 pg/mL, a prognosticating value associated with respiratory failure in patients with COVID-19 [11]. Baseline levels of other important cytokines of interest were also variably elevated. In the early stages of the COVID-19 pandemic, a study by Huang et al. revealed that elevated circulating levels of IL-6 were associated with clinical deterioration and the need for critical care support, suggesting that IL-6 could potentially serve as a target for intervention [4, 32]. Although the prognostic value of TNF-α and IL-1β as therapeutic targets in COVID-19 is not known [33,34,35], they are important makers of disease severity in other infectious and inflammatory conditions [36,37,38]. Despite marked cytokine levels at presentation, the overall clinical outcomes of participants in both our interventional and control groups were similar, and we were not able to detect any significant interaction with RIC.
The drivers of the severe hypercytokinaemia noted during fatal COVID-19 infections remain ill-understood and are likely influenced by both host- and virus-related factors [33, 39]. This is important because, in our study, there were significant differences in baseline characteristics, comorbidities, and measures of disease severity between recruiting centres. In particular, participants from South Africa had a higher frequency of the metabolic syndrome (dyslipidaemia, type 2 diabetes, and obesity) and HIV infection compared to participants in Brazil. Significant differences were also noted in baseline oxygenation and symptom onset between participants at both sites. Those from SA presented earlier with lower oxygen saturation levels compared to participants from Brazil. Although these differences may have influenced both the cytokine profile and the cytoprotective signalling induced by RIC, no significant interactions were found between baseline characteristics or clinical variables and the impact of RIC.
Among patients with COVID-19, several phases of the disease are described, each associated with different cytokine release profiles [40,41,42]. This is important because the marked variation in measured cytokines both at baseline and throughout the study may be accounted for by the fact that the phase at presentation was not known. We attempted to account for within-participant, between-participants, and between-site differences in cytokine profiles by using linear mixed-effects modelling to analyze the impact of RIC versus sham over time. However, whether this was adequate to mitigate against the impact of this phase effect is not clear. Therefore, enrolling participants in different phases of their disease may in part explain our findings. While the aforementioned limitations may not have fully unmasked a positive signal from RIC, our study has been very helpful in identifying and validating potential pitfalls for success as described by Bell et al. [19] and highlights the need to further investigate the easily accessible and cost-effective benefits of RIC in patients with COVID-19. Given the involvement of ongoing inflammation with possible cytokine elevation in patients with long COVID-19 and the paucity of specific treatments for this syndrome, it may be interesting to study the potential benefit of RIC in this population [ 43. ].
Limitations
This study has several limitations that need to be considered when evaluating the findings. Firstly, as a pilot study, the sample size could not be calculated a priori, limiting the statistical power for some outcomes. In addition, as we did not set any scale to determine the severity of non-critically ill patients enrolled at hospital admission, many participants displayed a wide variation in symptom onset. Furthermore, our study was designed and conducted during an era of the pandemic when the optimal treatment strategy for patients with COVID-19 was rapidly evolving. For this reason, corticosteroid initiation was not mandatory before enrolment and left to the treating physician’s discretion, and as a result, varied significantly between sites. These factors may have contributed to the extensive variation in cytokine concentrations and made it difficult to evaluate the effect of RIC. This later point is important as it clearly affected our secondary endpoint, which was to test RIC as an adjunct to standard of care. In addition, a critical limitation was encountered during cytokine analysis where a substantial amount of cytokine concentrations across hospitalization were found undetectable as they fell below the standardized kit’s detection level and, as a result, could not be included in the analysis. Finally, the study was carried out in the early phase of the pandemic with mostly unvaccinated participants. Therefore, care should be taken when extrapolating these findings to contemporary patients vaccinated and exposed to different strains of SARS-CoV-2.
Conclusion
Compared to sham, RIC did not reduce the in-hospital inflammatory cytokine cascade associated with moderate-and-severe COVID-19 and did not mitigate clinical deterioration. The findings from the “RIC in COVID-19” trial have highlighted the need for further research into the understanding of RIC and the dysregulated hyperinflammatory spectrum induced by SARS-CoV-2.
Data Availability
Requests for data collected for the study can be made to the co-corresponding author and will be considered individually. Additional related documents are immediately available (e.g., study protocol and informed consent form) and can be requested from the co-corresponding author.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- BIC:
-
Bayesian Information Criterion
- COVID-19:
-
Coronavirus 2019
- CRF:
-
Case report form
- CRP:
-
C-reactive protein
- HIV:
-
Human immunodeficiency virus
- IL:
-
Interleukin
- IFN:
-
Interferon
- LMIC:
-
Low- to middle-income countries
- MODS:
-
Multiple organ dysfunction syndrome
- RIC:
-
Remote ischaemic conditioning
- SA:
-
South Africa
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TNF-α:
-
Tumor necrosis factor-α
- UCL:
-
University College London
References
WHO COVID-19 Dashboard. Geneva: World Health Organization. 2020. Available from: https://covid19.who.int/. Accessed 14 Feb 2022.
Pearce L, Davidson SM, Yellon DM. The cytokine storm of COVID-19: a spotlight on prevention and protection. Expert Opin Ther Targets. 2020;24(8):723–30.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506.
Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–62.
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `cytokine storm’ in COVID-19. J Infect. 2020;80(6):607–13.
Akbari H, Tabrizi R, Lankarani KB, Aria H, Vakili S, Asadian F, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258: 118167.
Manson JJ, Crooks C, Naja M, Ledlie A, Goulden B, Liddle T, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. The Lancet Rheumatol. 2020;2(10):e594–602.
Harrison C. Focus shifts to antibody cocktails for COVID-19 cytokine storm. Nat Biotechnol. 2020;38(8):905–8.
Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30.
Santa Cruz A, Mendes-Frias A, Oliveira AI, Dias L, Matos AR, Carvalho A, et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front Immunol. 2021;12: 613422.
Abbasi-Oshaghi E, Mirzaei F, Farahani F, Khodadadi I, Tayebinia H. Diagnosis and treatment of coronavirus disease 2019 (COVID-19): laboratory, PCR, and chest CT imaging findings. Int J Surg. 2020;79:143–53.
Levy DE, Garcia-Sastre A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 2001;12(2–3):143–56.
Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Garcia Charello Ossoski R, et al. IFN-gamma is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289:198171.
Kim YH, Yoon DW, Kim JH, Lee JH, Lim CH. Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J Inflamm (Lond). 2014;11(16):16.
Honda T, He Q, Wang F, Redington AN. Acute and chronic remote ischemic conditioning attenuate septic cardiomyopathy, improve cardiac output, protect systemic organs, and improve mortality in a lipopolysaccharide-induced sepsis model. Basic Res Cardiol. 2019;114(3):15.
Joseph B, Khalil M, Hashmi A, Hecker L, Kulvatunyou N, Tang A, et al. Survival benefits of remote ischemic conditioning in sepsis. J Surg Res. 2017;213:131–7.
Pearce L, Davidson SM, Yellon DM. Does remote ischaemic conditioning reduce inflammation? A focus on innate immunity and cytokine response. Basic Res Cardiol. 2021;116(1):12.
Bell RM, Basalay M, Botker HE, Beikoghli Kalkhoran S, Carr RD, Cunningham J, et al. Remote ischaemic conditioning: defining critical criteria for success-report from the 11th Hatter Cardiovascular Workshop. Basic Res Cardiol. 2022;117(1):39.
Gaspar A, Lourenco AP, Pereira MA, Azevedo P, Roncon-Albuquerque R Jr, Marques J, et al. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol. 2018;113(3):14.
Yellon DM, Ackbarkhan AK, Balgobin V, Bulluck H, Deelchand A, Dhuny MR, et al. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol. 2015;65(25):2764–5.
Cour M, Gomez L, Mewton N, Ovize M, Argaud L. Postconditioning: from the bench to bedside. J Cardiovasc Pharmacol Ther. 2011;16(2):117–30.
Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ Res. 2013;113(4):439–50.
Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13(4):193–209.
Hadebe N, Cour M, Lecour S. The SAFE pathway for cardioprotection: is this a promising target? Basic Res Cardiol. 2018;113(2):9.
Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011;8(11):619–29.
Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65(2):177–95.
Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
Davidson SM, Lukhna K, Gorog DA, Salama AD, Castillo AR, Giesz S, et al. RIC in COVID-19-a clinical trial to investigate whether remote ischemic conditioning (RIC) can prevent deterioration to critical care in patients with COVID-19. Cardiovasc Drugs Ther. 2022;36(5):925–30.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–7.
Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(4):799-807 e9.
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–9.
Chen XY, Yan BX, Man XY. TNFalpha inhibitor may be effective for severe COVID-19: learning from toxic epidermal necrolysis. Ther Adv Respir Dis. 2020;14:1753466620926800.
Cremer PC, Sheng CC, Sahoo D, Dugar S, Prada RA, Wang TKM, et al. Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation. Eur Heart J Open. 2021;1(1):oeab002.
Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369(8):754–62.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Lin PL, Plessner HL, Voitenok NN, Flynn JL. Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc. 2007;12(1):22–5.
Darif D, Hammi I, Kihel A, El Idrissi SI, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153: 104799.
Griffin DO, Brennan-Rieder D, Ngo B, Kory P, Confalonieri M, Shapiro L, et al. The importance of understanding the stages of COVID-19 in treatment and trials. AIDS Rev. 2021;23(1):40–7.
Cappanera S, Palumbo M, Kwan SH, Priante G, Martella LA, Saraca LM, et al. When does the cytokine storm begin in COVID-19 patients? A quick score to recognize it. J Clin Med. 2021;10(2):297
Gupta G, Shareef I, Tomar S, Kumar MSN, Pandey S, Sarda R, et al. Th1/Th2/Th17 cytokine profile among different stages of COVID-19 infection. Natl Acad Sci Lett. 2022;45(4):363–9.
Gyöngyösi M, Alcaide P, Asselbergs FW, Brundel BJJM, Camici GG, da Costa Martins P, et al. (2022) Long COVID and the cardiovascular system—elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc Res. cvac115. https://doi.org/10.1093/cvr/cvac115
Acknowledgements
We would like to thank the “RIC in COVID-19” research team. We would also like to thank the patients and their families for participating in our study and all healthcare workers for their hard work and dedication.
Funding
Funding for this study was generously provided by grants from the Thompson Family Trust, the Hatter Cardiovascular Institute-UCL, the Mancherje-Potash Foundation, the Fundação de Apoio a Pesquisa do Estado de São Paulo (FAPESP), and the SAMRC Self-Initiated Research Grant. In addition, KL has received additional funding through the UCT Start-up Emerging Researcher Award (SERA) and Edith Sorell’s cardiovascular research fellowship. AS was supported by a Research Career Awards grant from the Brazilian National Research Council (CNPq) (grant number 304257/2021–4).
Author information
Authors and Affiliations
Contributions
KL, AS, MN, and DY wrote the first draft of the manuscript. HG analyzed the data. All authors vouch for the data and the analysis, have contributed to the writing of the paper, and participated in the decision to publish the paper.
Corresponding author
Ethics declarations
Ethics Approval
All aspects of the study conduct, including the consent process and collection of the assessments used in this analysis, were approved by institutional ethics review boards from each site.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kishal Lukhna is the first author.
Mpiko Ntsekhe, Andrei C. Sposito, and Derek M Yellon are the joint senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lukhna, K., do Carmo, H.R.P., Castillo, A.R. et al. Effect of Remote Ischaemic Conditioning on the Inflammatory Cytokine Cascade of COVID-19 (RIC in COVID-19): a Randomized Controlled Trial. Cardiovasc Drugs Ther 38, 433–445 (2024). https://doi.org/10.1007/s10557-022-07411-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-022-07411-2