Abstract
Purpose
Ticagrelor and dapagliflozin can suppress the activation of the NOD-like receptor 3 (NLRP3)-inflammasome and activate AMP-activated protein kinase (AMPK). The anti-inflammatory effects of dapagliflozin has been shown to depend on AMPK activation. Dapagliflozin and ticagrelor have been shown to have additive effects on the progression of diabetic cardiomyopathy in BTBR ob/ob mice with type-2 diabetes. We assessed whether dapagliflozin and ticagrelor have additive effects on the activation of the NLRP3-inflammasome and the progression of diabetic nephropathy in mice with type-2 diabetes.
Methods
Eight-week-old BTBR received either no-drug, dapagliflozin (1.5 mg/kg/d), ticagrelor (100 mg/kg/d), or their combination for 12 weeks. Blood was assessed weekly for glucose and urine for glucose and albumin. After 12 weeks, blood creatinine, cystatin C, inflammasome activation, and insulin were assessed by ELISA. Renal cortex samples were assessed by hematoxylin and eosin and periodic acid-Schiff staining. RT-PCR and immunoblotting were used to evaluate fibrosis and the activation of Akt, AMPK and the inflammasome.
Results
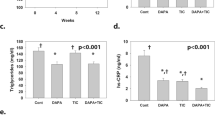
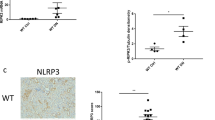
Both ticagrelor and dapagliflozin reduced serum creatinine and cystatin C levels and urinary albumin. Both drugs attenuated the increase in glomerular area and mesangial matrix index. Both drugs decreased collagen-1 and collagen-3 expression and the activation of the NLRP3-inflammasome. Both drugs increased P-AMPK levels, but only dapagliflozin increased P-Akt levels. Overall, the protective effects of dapagliflozin and ticagrelor were additive.
Conclusions
Dapagliflozin and ticagrelor attenuated the progression of diabetic nephropathy in BTBR ob/ob mice with additive effects of the combination. This was associated with AMPK activation and reduced activation of the NLRP3 inflammasome, whereas only dapagliflozin increased Akt activation.






Similar content being viewed by others
Data Availability
Original research data will be available upon request.
References
Thomas B. The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diab Rep. 2019;19(4):18.
Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev. 2015;4(2):28–33.
Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106(2):26–31.
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158. https://doi.org/10.1001/jamacardio.2020.4511.
Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–54.
Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606–17.
Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46.
McMurray JJV, Wheeler DC, Stefansson BV, Jongs N, Postmus D, Correa-Rotter R, et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2021;143(5):438–448. https://doi.org/10.1161/CIRCULATIONAHA.120.051675.
Birnbaum Y, Bajaj M, Yang HC, Ye Y. Combined SGLT2 and DPP4 inhibition reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic nephropathy in mice with type 2 diabetes. Cardiovasc Drugs Ther. 2018;32(2):135–45.
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 Inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31(2):119–32.
Dai H, Liu Q, Liu B. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res. 2017;2017:2615286.
Eisenreich A, Leppert U. Update on the protective renal effects of metformin in diabetic nephropathy. Curr Med Chem. 2017;24(31):3397–412.
Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309–20.
Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2-dependent effect. Arterioscler Thromb Vasc Biol. 2014;34(9):2078–85.
Birnbaum Y, Tran D, Chen H, Nylander S, Sampaio LC, Ye Y. Ticagrelor improves remodeling, reduces apoptosis, inflammation and fibrosis and increases the number of progenitor stem cells after myocardial infarction in a rat model of ischemia reperfusion. Cell Physiol Biochem. 2019;53(6):961–81.
Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc Drugs Ther. 2016;30(6):539–50.
Chen H, Tran D, Yang HC, Nylander S, Birnbaum Y, Ye Y. Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: an AMPK-mTOR interplay. Cardiovasc Drugs Ther. 2020;34(4):443–61.
Vilahur G, Gutierrez M, Casani L, Lambert C, Mendieta G, Ben-Aicha S, et al. P2Y12 antagonists and cardiac repair post-myocardial infarction: global and regional heart function analysis and molecular assessments in pigs. Cardiovasc Res. 2018;114(14):1860–70.
Uil M, Butter LM, Claessen N, Larsen PW, Florquin S, Roelofs J. Platelet inhibition by ticagrelor is protective against diabetic nephropathy in mice. FASEB J. 2020;34(10):13750–13761. https://doi.org/10.1096/fj.202000897R.
Li X, Li Y, Shen K, Li H, Bai J. The protective effect of ticagrelor on renal function in a mouse model of sepsis-induced acute kidney injury. Platelets. 2019;30(2):199–205.
Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, et al. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019;394(10204):1169–80.
Akerblom A, Wallentin L, Siegbahn A, Becker RC, Budaj A, Horrow J, et al. Outcome and causes of renal deterioration evaluated by serial cystatin C measurements in acute coronary syndrome patients – results from the PLATelet inhibition and patient Outcomes (PLATO) study. Am Heart J. 2012;164(5):728–34.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Ayinde KS, Olaoba OT, Ibrahim B, Lei D, Lu Q, Yin X, et al. AMPK allostery: a therapeutic target for the management/treatment of diabetic nephropathy. Life Sci. 2020;261:118455.
Kume S, Thomas MC, Koya D. Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes. 2012;61(1):23–9.
Eid S, Boutary S, Braych K, Sabra R, Massaad C, Hamdy A, et al. mTORC2 signaling regulates Nox4-induced podocyte depletion in diabetes. Antioxid Redox Signal. 2016;25(13):703–19.
Habib SL, Yadav A, Kidane D, Weiss RH, Liang S. Novel protective mechanism of reducing renal cell damage in diabetes: activation AMPK by AICAR increased NRF2/OGG1 proteins and reduced oxidative DNA damage. Cell Cycle. 2016;15(22):3048–59.
Yang D, Livingston MJ, Liu Z, Dong G, Zhang M, Chen JK, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci. 2018;75(4):669–88.
Luo X, Deng L, Lamsal LP, Xu W, Xiang C, Cheng L. AMP-activated protein kinase alleviates extracellular matrix accumulation in high glucose-induced renal fibroblasts through mTOR signaling pathway. Cell Physiol Biochem. 2015;35(1):191–200.
Tuttle KR. Back to the future: glomerular hyperfiltration and the diabetic kidney. Diabetes. 2017;66(1):14–6.
Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 Inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis. 2018;72(2):267–77.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–72.
Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014;63(23):2503–9.
Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35(8):1805–14.
Lizcano JM, Alessi DR. The insulin signalling pathway. Curr Biol. 2002;12(7):R236–8.
Birnbaum Y, Nanhwan MK, Ling S, Perez-Polo JR, Ye Y, Bajaj M. PTEN upregulation may explain the development of insulin resistance and type 2 diabetes with high dose statins. Cardiovasc Drugs Ther. 2014;28(5):447–57.
Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2(3):220–3.
Yang K, Chen Z, Gao J, Shi W, Li L, Jiang S, et al. The key roles of GSK-3beta in regulating mitochondrial activity. Cell Physiol Biochem. 2017;44(4):1445–59.
Lin CL, Cheng H, Tung CW, Huang WJ, Chang PJ, Yang JT, et al. Simvastatin reverses high glucose-induced apoptosis of mesangial cells via modulation of Wnt signaling pathway. Am J Nephrol. 2008;28(2):290–7.
Mathur A, Pandey VK, Kakkar P. Activation of GSK3beta/beta-TrCP axis via PHLPP1 exacerbates Nrf2 degradation leading to impairment in cell survival pathway during diabetic nephropathy. Free Radic Biol Med. 2018;120:414–24.
Paeng J, Chang JH, Lee SH, Nam BY, Kang HY, Kim S, et al. Enhanced glycogen synthase kinase-3beta activity mediates podocyte apoptosis under diabetic conditions. Apoptosis. 2014;19(12):1678–90.
Funding
The study was funded by an investigator initiated grant from AstraZeneca and the John S. Dunn Chair in Cardiology Research and Education.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, experiments, and data collection were performed by Huan Chen, Dat Tran, and Yumei Ye. Data analysis was performed by Yumei Ye and Yochai Birnbaum. Figures were made by Yochai Birnbaum. The first draft of the manuscript was written by Yochai Birnbaum, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experimental designs and animal care were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996) and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch.
Conflict of Interest
Huan Chen,none; Dat Tran,none; Sven Nylander is an employee of Astra Zeneca; Yochai Birnbaum, Research Grant by Astra Zeneca; Yumei Ye, Research Grant by Astra Zeneca.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Birnbaum, Y., Chen, H., Tran, D. et al. Ticagrelor and Dapagliflozin Have Additive Effects in Ameliorating Diabetic Nephropathy in Mice with Type-2 Diabetes Mellitus. Cardiovasc Drugs Ther 36, 829–840 (2022). https://doi.org/10.1007/s10557-021-07222-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07222-x