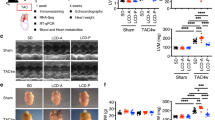
Abstract
Purpose
To evaluate the effectiveness of vitamin D3 supplementation, in secondary prevention, on cardiac remodeling and function, as well as lipid profile, in a mouse model of diet-induced type 2 diabetes.
Methods
Mice were fed a high fat and sucrose diet for 10 weeks. Afterward, diet was maintained for 15 more weeks and two groups were formed, with and without cholecalciferol supplementation. A control group was fed with normal chow. Glucose homeostasis and cardiac function were assessed at baseline and at the 10th and 24th weeks. Animals were killed at the 10th and 25th weeks for plasma and cardiac sample analysis. Cardiac lipid profile was characterized by LC-MS/MS.
Results
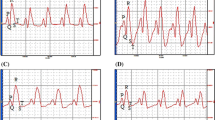
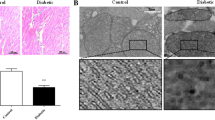
After 10 weeks of diet, mice exhibited pre-diabetes, mild left ventricle hypertrophy, and impaired longitudinal strain, but preserved myocardial circumferential as well as global diastolic and systolic cardiac function. After 15 more weeks of diet, animals presented with well-established type 2 diabetes, pathological cardiac hypertrophy, and impaired regional myocardial function. Cholecalciferol supplementation had no effect on glucose homeostasis but improved cardiac remodeling and regional myocardial function. After 25 weeks, non-supplemented mice exhibited increased myocardial levels of ceramides and diacylglycerol, both of which were normalized by vitamin D3 supplementation.
Conclusion
This work brought to light the beneficial effects of cholecalciferol supplementation, in secondary prevention, on cardiac remodeling and function in a mouse model of diet-induced type 2 diabetes. Those cardioprotective effects may be, at least in part, attributed to the modulation of myocardial levels of lipotoxic species by vitamin D.





Similar content being viewed by others
Availability of Data and Material
Data from this work are available upon reasonable request to corresponding author.
References
Waddingham MT, Edgley AJ, Tsuchimochi H, Kelly DJ, Shirai M, Pearson JT. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World J Diabetes. 2015;6:943–60.
van der Meer RW, Rijzewijk LJ, Diamant M, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29:1516–22.
Hammer S, Snel M, Lamb HJ, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–12.
Christoffersen C, Bollano E, Lindegaard MLS, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–90.
Liu L, Shi X, Bharadwaj KG, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–23.
Glenn DJ, Wang F, Nishimoto M, et al. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension. 2011;57:216–22.
Park S-Y, Cho Y-R, Kim H-J, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in c57bl/6 mice. Diabetes. 2005;54:3530–40.
Ouwens DM, Diamant M, Fodor M, et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia. 2007;50:1938–48.
Ternacle J, Wan F, Sawaki D, et al. Short-term high-fat diet compromises myocardial function: a radial strain rate imaging study. Eur Heart J Cardiovasc Imaging. 2017;18:1283–91.
Calligaris SD, Lecanda M, Solis F, et al. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 2013;8:e60931.
Ng ACT, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–401.
Zhang X, Wei X, Liang Y, Liu M, Li C, Tang H. Differential changes of left ventricular myocardial deformation in diabetic patients with controlled and uncontrolled blood glucose: a three-dimensional speckle-tracking echocardiography–based study. J Am Soc Echocardiogr. 2013;26:499–506.
Hankiewicz JH, Banke NH, Farjah M, Lewandowski ED. Early impairment of transmural principal strains in the left Ventricular Wall after short-term, high-fat feeding of mice predisposed to cardiac steatosis. Circ Cardiovasc Imaging. 2010;3:710–7.
Xiang W, Kong J, Chen S, et al. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol-Endocrinol Metab. 2005;288:E125–32.
Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–9.
Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the T-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology. 2008;149:558–64.
Afzal S, Bojesen SE, Nordestgaard BG. Low 25-Hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and Metaanalysis. Clin Chem. 2013;59:381–91.
Chen Y, Zhao C-T, Zhen Z, Wong A, Tse HF, Yiu KH. Association of myocardial dysfunction with vitamin D deficiency in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2014;28:286–90.
Heidari B, Nargesi AA, Hafezi-Nejad N, et al. Assessment of serum 25-hydroxy vitamin D improves coronary heart disease risk stratification in patients with type 2 diabetes. Am Heart J. 2015;170:573–579.e5.
Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol. 2006;41:350–9.
Lee T-W, Kao Y-H, Lee T-I, Chang C-J, Lien G-S, Chen Y-J. Calcitriol modulates receptor for advanced glycation end products (RAGE) in diabetic hearts. Int J Cardiol. 2014;173:236–41.
Wei H, Qu H, Wang H, et al. 1,25-Dihydroxyvitamin-D3 prevents the development of diabetic cardiomyopathy in type 1 diabetic rats by enhancing autophagy via inhibiting the β-catenin/TCF4/GSK-3β/mTOR pathway. J Steroid Biochem Mol Biol. 2017;168:71–90.
Mazzone G, Morisco C, Lembo V, et al. Dietary supplementation of vitamin D prevents the development of western diet-induced metabolic, hepatic and cardiovascular abnormalities in rats. United European Gastroenterol J. 2018;6:1056–64.
Marziou A, Philouze C, Couturier C, et al. Vitamin D supplementation improves adipose tissue inflammation and reduces hepatic steatosis in obese C57BL/6J mice. Nutrients. 2020;12:342.
Bonnet L, Karkeni E, Couturier C, et al. Gene expression pattern in response to cholecalciferol supplementation highlights Cubilin as a major protein of 25(OH)D uptake in adipocytes and male mice white adipose tissue. Endocrinology. 2018;159:957–66.
Enomoto M, Ishizu T, Seo Y, et al. Myocardial dysfunction identified by three-dimensional speckle tracking echocardiography in type 2 diabetes patients relates to complications of microangiopathy. J Cardiol. 2016;68:282–7.
Brainard RE, Watson LJ, DeMartino AM, et al. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS One. 2013;8:e83174.
Lee T-I, Kao Y-H, Chen Y-C, Tsai W-C, Chung C-C, Chen Y-J. Cardiac metabolism, inflammation, and peroxisome proliferator-activated receptors modulated by 1,25-dihydroxyvitamin D3 in diabetic rats. Int J Cardiol. 2014;176:151–7.
Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–4.
Park T-S, Hu Y, Noh H-L, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–12.
Zhang L, Ussher JR, Oka T, Cadete VJJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res. 2011;89:148–56.
Parra V, Moraga F, Kuzmicic J, et al. Calcium and mitochondrial metabolism in ceramide-induced cardiomyocyte death. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2013;1832:1334–44.
Simon JN, Chowdhury SAK, Warren CM, et al. Ceramide-mediated depression in cardiomyocyte contractility through PKC activation and modulation of myofilament protein phosphorylation. Basic Res Cardiol. 2014;109. https://doi.org/10.1007/s00395-014-0445-6
Wang H, Sreenivasan U, Gong D-W, et al. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction1. J Lipid Res. 2013;54:953–65.
Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA. Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discov Today Dis Mech. 2010;7:e135–43.
Shea BS, Tager AM. Sphingolipid regulation of tissue fibrosis. Open Rheumatol J. 2012;6:123–9.
Chen C-L, Lin C-F, Chang W-T, Huang W-C, Teng C-F, Lin Y-S. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–74.
Perry RA, Brown LA, Haynie WS, et al. Cardiac hypertrophy in sarcopenic obese C57BL/6J mice is independent of Akt/mTOR cellular signaling. Exp Gerontol. 2018;111:122–32.
Farhangi MA, Nameni G, Hajiluian G, Mesgari-Abbasi M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat- diet induced obese rats. BMC Cardiovasc Disord. 2017;17(1):161.
Acknowledgments
This work was supported by the Platform 3A, funded by the European Regional Development Fund, the French Ministry of Research Higher Education and Innovation, the Provence-Alpes-Côte-d’Azur region, the Departmental Council of Vaucluse and the Urban Community of Avignon.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
CP, JCM, CR, AM, JFL, and PO contributed to the conception and design of the study; CP, JCM, CR, AM, CC, TB, JA, CD, BJ, NG, CR, SG, JFL, and PO were involved in the data acquisition, analysis, and interpretation; CP, JFL, and PO were involved in drafting the article or revising it critically for important intellectual content.
All authors reviewed/edited the manuscript and approved the final version.
PO is the guarantor of the present work.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics Approval
All experimental procedures meet European parliamentary guidelines 2010/63/EU (N°CEEA-00322.03) and were approved by the local ethics committee (N°2,017,071,209,197,796).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Philouze, C., Martin, JC., Riva, C. et al. Vitamin D3 Supplementation Alleviates Left Ventricular Dysfunction in a Mouse Model of Diet-Induced Type 2 Diabetes: Potential Involvement of Cardiac Lipotoxicity Modulation. Cardiovasc Drugs Ther 36, 245–256 (2022). https://doi.org/10.1007/s10557-021-07143-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07143-9