Abstract
Background
In patients with acute coronary syndrome (ACS), angiotensin-converting enzyme (ACE) inhibitors are preferred over angiotensin receptor blockers (ARBs). However, in a recent pilot study, treatment with ACE inhibitors was associated with increased platelet reactivity compared to ARBs. Therefore, we sought to investigate the impact of renin-angiotensin-aldosterone system (RAAS) blockade with ACE inhibitors and ARBs on platelet aggregation in patients with ACS undergoing percutaneous coronary intervention.
Methods
On-treatment residual platelet reactivity in response to arachidonic acid (AA), adenosine diphosphate (ADP), SFLLRN, AYPGKF, and collagen was assessed by multiple electrode aggregometry (MEA) in 197 ACS patients on dual antiplatelet therapy (DAPT) with aspirin and either prasugrel or ticagrelor.
Results
One hundred sixty-five (83.7%) patients were treated with ACE inhibitors, 32 (16.3%) with ARBs. On-treatment residual AA- and ADP-inducible platelet reactivity was significantly higher in patients with ACE inhibitors (both p < 0.05). Likewise, SFLLRN was significantly higher in patients with ACE inhibitors (p = 0.036) and there was a trend for higher AYPGKF- and collagen-inducible platelet reactivity (p = 0.053 and p = 0.082). The incidence of high on-treatment residual platelet reactivity AA was significantly higher in patients with ACE inhibitors (52 [31.5%] vs. 3 [9.4%] patients; p = 0.019).
Conclusion
ACE inhibitors are associated with increased on-treatment residual platelet reactivity in ACS patients with potent DAPT. Further clinical trials are needed to elucidate the role of RAAS blockade with ACE inhibitors and ARBs in ACS patients treated according to current standards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is the antithrombotic standard regimen for patients presenting with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI) [1,2,3]. Prasugrel and ticagrelor are newer adenosine diphosphate (ADP) P2Y12 receptor antagonists that have been shown to be superior compared to clopidogrel in reducing adverse cardiovascular outcomes in ACS due to faster, stronger, and more consistent inhibition of ADP-induced platelet activation [4, 5]. However, atherothrombotic events still impair the prognosis of many patients with ACS despite state-of-the-art antiplatelet therapy [6]. While high on-treatment residual platelet reactivity (HRPR) to ADP is a rare phenomenon in patients treated with prasugrel or ticagrelor [7], subsequent ischemic events in patients receiving the novel P2Y12 antagonists may in part be attributable to platelet activation via other, non-inhibited pathways [8,9,10]. In addition, previous studies have identified several concomitant pharmacological therapies potentially attenuating the antiplatelet effects of clopidogrel [11,12,13,14].
According to current guidelines, renin-angiotensin-aldosterone system (RAAS) blockade with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) should be considered in all patients presenting with ST-elevation myocardial infarction (STEMI) and is recommended for all ACS patients suffering from either arterial hypertension, chronic kidney disease, diabetes, or reduced left-ventricular ejection fraction [2, 3]. Moreover, ARBs should only prescribe in case of intolerance against ACE inhibitors [2, 3].
However, in a recent pilot-analysis by Helten et al. including patients with an indication for RAAS blockade (arterial hypertension or heart failure), ACE inhibitors were associated with increased platelet surface expression of protease-activated receptor-1 (PAR-1) and enhanced SFLLRN (=thrombin receptor activating peptide [TRAP])-inducible platelet reactivity compared to ARBs [15].
Considering these recent data, we sought to assess the impact of RAAS blockade with ACE inhibitors or ARBs on platelet aggregation in ACS patients receiving either prasugrel or ticagrelor following acute PCI.
Methods
Patient Population
The study population consisted of 197 ACS patients on daily aspirin (100 mg/day), and either prasugrel (10 mg/day) or ticagrelor (180 mg/day) therapy. All study patients were of Caucasian ethnicity. Blood sampling was performed 72 h after acute PCI with stent implantation. Due to the short half-life of unfractionated heparin, all patients were free of heparin from PCI at the time of blood sampling [16].
Exclusion criteria were a known P2Y12 inhibitor or aspirin intolerance (manifested as allergic reactions or gastrointestinal bleeding); a therapy with vitamin K antagonists (phenprocoumon, acenocoumarol, warfarin), rivaroxaban, apixaban, dabigatran, or edoxaban; treatment with nonsteroidal anti-inflammatory drugs, ticlopidine, or dipyridamole; known bleeding disorders; severe hepatic failure; known qualitative defects in platelet function; heparin-induced thrombocytopenia; malignant myeloproliferative disorders; a platelet count < 100,000 or > 450,000/μL; a hematocrit < 30%; and a major surgical procedure within 1 week before enrollment.
Blood Sampling
Blood was drawn by aseptic venipuncture from an antecubital vein using a butterfly needle (21 gauge, 0.8 × 19 mm; Greiner Bio-One, Kremsmünster, Austria) 72 h after PCI, as described previously [9]. To avoid procedural deviations, blood sampling was performed by the same physician applying a light tourniquet, which was immediately released, and the samples were mixed by gently inverting the tubes. The initial 3 mL of blood was discarded to reduce periprocedural platelet activation. Afterwards, blood was drawn into hirudin-coated tubes (Roche Diagnostics, Mannheim, Germany) for multiple electrode aggregometry (MEA).
Multiple Electrode Aggregometry
Whole blood impedance aggregometry was performed using the Multiplate analyzer (Roche Diagnostics) as previously described [10]. In brief, hirudin-anticoagulated whole blood was diluted 1:2 with 0.9% NaCl solution and stirred in the test cuvettes for 3 min at 37 °C. Thereafter, arachidonic acid (AA; 0.5 mM), ADP (6.4 μM), collagen (2.7 μg/mL), SFLLRN (PAR-1 agonist, 32 μM), or AYPGKF (PAR-4 agonist, 645 μM, all from Roche Diagnostics) was added and aggregation was recorded for 6 min. Titration experiments were carried out, increasing the dosages of SFLLRN and AYPGKF, respectively, until both agonists induced platelet aggregation > 60 aggregation units (AU) by MEA, but less than maximal response in healthy Caucasian individuals (n = 30). The determined dosages corresponded to the concentrations recommended by the manufacturer. The interaction of activated platelets with the electrode led to an increase of impedance, which was detected for each sensor unit separately and transformed to aggregation units (AU) that were plotted against time. The AU at 6 min were used for calculations. One AU corresponds to 10 AU*min (area under the curve of AU).
Statistical Analysis
All continuous variables are expressed as median (interquartile range [IQR]). Categorical variables are given as number (%). Continuous variables were compared by Mann-Whitney U test for independent samples. χ2 test or Fisher’s exact test was performed for comparison of categorical variables, as appropriate. Multivariable linear regression analyses using a backward elimination algorithm with a p value ≤ 0.1 for removal were used to adjust for patient characteristics. Adjustment was performed for the following variables: age, sex, body mass index, hypertension, hyperlipidemia, diabetes, active smoking, peripheral artery disease, prior myocardial infarction, prior stroke or transient ischemic attack, number of affected coronary vessels, type of P2Y12 inhibitor, use of statins, beta blockers, or proton pump inhibitors. All statistical tests were 2-tailed and a p value < 0.05 was considered significant. All statistical analyses and figures were generated with Statistical Package for Social Sciences (IBM SPSS version 24, Armonk, NY, USA) and R 3.6.3.
Results
In total, 197 patients were eligible for analysis. Of all patients, 165 (83.7%) patients were treated with an ACE inhibitor, 32 (16.3%) with an ARB. Median age was 57 (IQR 49–66) years, and 36 (18.3%) were female. Prasugrel was prescribed to 113 (57.4%), and ticagrelor to 84 (42.6%) patients. Clinical, laboratory, and procedural characteristics of the overall study population and stratified for patients with ARBs and ACE inhibitors are presented in Table 1.
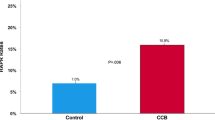
On-treatment residual AA- and ADP-inducible platelet reactivity was significantly higher in patients with ACE inhibitors as compared to patients with ARBs (AA: 17 AU [12–22] vs. 13 AU [7–18], p = 0.006; ADP: 20 AU (16–24) vs. 16 AU (11–20), p < 0.001; Table 2, Fig. 1). Likewise, SFLLRN-inducible platelet reactivity was significantly higher in patients with ACE inhibitors (67 AU [50–84] vs. 60 AU [41–76], p = 0.036; Table 2, Fig. 1), and there was a trend for higher AYPGKF- and collagen-inducible platelet reactivity (AYPGKF: 64 AU [47–82] vs. 58 AU [37–75], p = 0.053; collagen: 60 AU [35–77] vs. 50 AU [26–72], p = 0.082) in patients on ACE inhibitors. In multivariable linear regression analysis, ACE inhibitor therapy remained significantly associated with increased AA- and ADP-inducible platelet reactivity (both p < 0.05), whereas after adjustment, no association with SFLLRN-, AYPGKF-, and collagen-induced platelet reactivity was detectable (all p > 0.05).
Aggregation units (AU) by multiple electrode aggregometry (MEA) in response to AA (panel a), ADP (panel b), SFLLRN (panel c), AYPGKF (panel d), and collagen (panel e) stratified for patients with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). Cut-off values for high on-treatment residual platelet reactivity are indicated by the dashed lines. The boundaries of the box show the lower and upper quartile of data, and the line inside the box represents the median. Whiskers are drawn from the edge of the box to the highest and lowest values that are outside the box but within 1.5 times the box length
High on-treatment residual platelet reactivity (HRPR) to AA and ADP was defined according to previous studies showing an association between platelet aggregation by MEA and ischemic outcomes following PCI [17, 18]. The respective cut-off values were AU ≥ 21 and > 46 for MEA AA and MEA ADP, respectively. With use of these thresholds, the frequency of HRPR ADP was similar between patients with ACE inhibitors and ARBs (2 vs. 0 patients; p = 1.000), whereas the incidence of HRPR AA was significantly higher in patients on ACE inhibitors (52 [31.5%] vs. 3 [9.4%] patients; p = 0.019).
In prasugrel-treated patients, ADP-, AYPGKF-, and SFLLRN-inducible platelet reactivity was significantly higher in those receiving ACE inhibitors as compared to patients on ARBs (all p < 0.05; Table 3), and there was a trend towards higher AA- and collagen-inducible platelet reactivity (AA: p = 0.058; collagen: p = 0.054) in patients on ACE inhibitors. However, after adjustment, ACE inhibitors no longer were associated with agonist-inducible residual platelet reactivity in patients treated with prasugrel (all p > 0.05).
In patients on ticagrelor, residual AA-inducible platelet reactivity was numerically but not statistically and ADP-inducible reactivity significantly higher in patients with ACE inhibitors. SFLLRN-, AYPGKF-, and collagen-inducible platelet reactivity was similar in patients with ACE inhibitors and ARBs (Table 3). In multivariable regression analysis, ACE inhibitors remained associated with ADP-inducible platelet reactivity (p = 0.002) but were not associated with AA-, SFLLRN-, AYPGKF-, and collagen-inducible residual platelet reactivity in patients on ticagrelor (all p > 0.05).
Discussion
This is the first study investigating the association of ACE inhibitors and ARBs with on-treatment residual platelet reactivity in ACS patients on potent DAPT undergoing PCI. Our main finding was that agonist-inducible platelet reactivity as assessed by MEA was higher in patients receiving ACE inhibitors as compared to patients receiving ARBs. Moreover, there was an increased incidence of HRPR AA in patients with ACE inhibitors.
The current antithrombotic therapy regimen following ACS with PCI includes aspirin and a potent P2Y12 inhibitor, either prasugrel or ticagrelor [2, 3]. Aspirin irreversibly acetylates a serine residue of cyclooxygenase (COX) 1 and COX 2, and inhibits thromboxane A2 (TXA2) generation by suppressing the synthesis of prostaglandin G2 and H2 [19]. Prasugrel and ticagrelor bind P2Y12, a G protein–coupled receptor for ADP, which is responsible for sustained amplification and stabilization of platelet aggregation [20]. However, platelets can be activated by various pathways and agonists, and residual platelet reactivity resulting in subsequent atherothrombotic events still impair the prognosis in many patients suffering from cardiovascular disease [20].
The beneficial effects of ACE inhibitors on vasoconstriction, cell growth, sodium and water retention, and sympathetic activation result from the suppression of angiotensin II formation, and consequently the downstream inhibition of the angiotensin II type 1 (AT1) and type 2 (AT2) receptors [21]. In contrast, ARBs specifically target the AT1 receptor and additionally trigger vasodilatation and natriuresis by facilitating the stimulation of the AT2 receptor [21]. In ACS patients, ACE inhibitors were associated with a small but significant reduction in 30-day mortality [22]. Moreover, in patients with ACS and systolic dysfunction, treatment with ARBs was not associated with a benefit in patient outcomes compared to ACE inhibitors [23, 24]. Finally, there are no clinical trials investigating ARBs in ACS patients with preserved left-ventricular function. Therefore, according to current guidelines, ACE inhibitors are the first choice; only if not tolerated ARBs shall be prescribed [2, 3]. However, in a recent propensity-matched analysis and a recent meta-analysis, ARBs demonstrated similar efficacy and superior safety compared to ACE inhibitors [25, 26]. Moreover, in line with our findings, a recent pilot study including 34 patients with arterial hypertension or heart failure treatment with an ACE inhibitor (ramipril) demonstrated significantly increased SFLLRN-inducible platelet reactivity already 4 h after treatment initiation, which remained elevated during therapy [15]. These findings were the result of reduced thrombin formation in response to ACE inhibitor therapy, which in turn enhanced PAR-1 surface expression on platelets leading to increased platelet reactivity. Interestingly, after switching to an ARB (candesartan) SFLLRN-inducible platelet reactivity and thrombin receptor, expression decreased significantly [15]. Of note, not all patients were on antiplatelet therapy (65% patients with aspirin, 44% patients with P2Y12 inhibitor) [15]. However, we previously demonstrated that platelet activation via the PAR1- and PAR-4 pathways remains active in many patients on DAPT, even in patients receiving the more potent P2Y12 inhibitors prasugrel and ticagrelor [9, 10, 27]. In our cohort with all patients on potent DAPT, SFLLRN-inducible platelet aggregation was higher in patients receiving ACE inhibitors. This is of particular interest, as in a former study, PAR-1-mediated platelet activation was associated with adverse outcomes in patients with peripheral angioplasty and stent implantation [28]. Moreover, treatment with vorapaxar (a PAR-1 antagonist) on top of DAPT significantly reduced the composite endpoint of myocardial infarction, stroke, and cardiovascular death in ACS patients in the TRACER trial, but also significantly increased the risk of bleeding including intracranial hemorrhage [29].
In a randomized controlled study including aspirin-treated patients with coronary artery disease (CAD; defined as < 50% stenosis in coronary angiography) comparing the impact RAAS blockade on platelet aggregation, irbesartan (an ARB) significantly reduced TXA2-induced platelet aggregation, whereas enalapril (an ACE inhibitor) had no effect on TXA2-induced platelet aggregation assessed by turbidometry [30]. As urinary prostaglandin E2 levels were unaffected, the authors suspected a COX-2 independent mechanism by either direct inhibitory effects of irbesartan or its active metabolites at the TXA2-receptor [30]. Likewise, in our cohort, we detected higher on-treatment residual AA- and ADP-inducible platelet reactivity by MEA in patients with ACE inhibitor therapy. Moreover, patients with ACE inhibitors had a higher incidence of HRPR AA compared to patients with ARBs. This is of great interest, as residual AA- and ADP-inducible platelet reactivity has repeatedly been associated with adverse ischemic outcomes in patients undergoing PCI and stenting on DAPT [17, 31,32,33].
It is noteworthy that no widely accepted threshold to identify patients with HRPR AA has been defined to date and higher thresholds than those used in our manuscript have been reported in the literature (MEA AA > 30 AU or > 40 AU, respectively) [34, 35]. However, these cut-offs derive from small analyses comparing healthy controls with patients on aspirin monotherapy and stable cardiovascular disease, and might not apply to patients with a recent acute ischemic event. Moreover, these cut-offs have not been validated in a clinical outcome trial. We therefore defined HRPR AA according to a recent report by Mayer et al. including patients undergoing PCI on DAPT, in which MEA AA < 21 AU was associated with a higher risk for death or stent thrombosis during the first year after PCI [17].
The use of prasugrel and ticagrelor has been associated with low ADP-inducible platelet reactivity in recent studies by others and us [9, 36, 37]. In line with these previous studies, HRPR ADP was only seen in 2 patients in the current study. In this regard, however, it should be kept in mind that all thresholds for HRPR in response to ADP were defined in clopidogrel-treated patients and may not apply to patients receiving prasugrel or ticagrelor. Furthermore, more potent platelet inhibition by the modern P2Y12 inhibitors (prasugrel and ticagrelor) was superior to clopidogrel in reducing thrombotic events and cardiovascular death, but increased the risk of bleeding complications [4, 5]. One may therefore speculate that more intense platelet inhibition as seen in patients with ARBs might result in a further clinical benefit regarding the reduction of ischemic outcomes in patients on potent P2Y12 inhibitors, potentially at the cost of an increased risk of bleeding complications.
ACE inhibitors are currently preferred over ARBs in the setting of ACS. However, these data derive from randomized clinical trials, which do not apply current standards including treatment with primary PCI for the majority of patients and potent DAPT with prasugrel and ticagrelor. Our data indicate a benefit of ARBs over ACE inhibitors with respect to on-treatment residual platelet reactivity in ACS patients with potent DAPT. Further clinical trials are needed to elucidate the role of RAAS blockade with ACE inhibitors and ARBS in ACS patients treated according to modern standards.
Limitations
Our data must be regarded as hypothesis-generating only and further investigations are needed, as this study cannot prove causality nor exclude other possible confounders. The present investigation should be interpreted with the following limitations in mind: Our study is not powered nor intended to provide clinical outcome data. Also, we measured platelet aggregation at only one time point using a single test system. Moreover, we did not perform in vivo analyses. However, MEA is a highly standardized platelet function test ensuring a good comparability of the obtained results with other laboratories, and platelet aggregation by MEA has repeatedly been linked to cardiovascular outcomes following PCI [17, 31].
Conclusion
ACE inhibitors are associated with increased on-treatment residual platelet reactivity in ACS patients on potent DAPT. Further clinical trials are needed to elucidate the role of RAAS blockade with ACE inhibitors and ARBs in ACS patients treated according to modern standards.
Data Availability
All participants were enrolled at the Department of Internal Medicine II of the Medical University of Vienna. The data underlying this article will be shared on reasonable request to the corresponding author.
References
Tscharre M, Michelson AD, Gremmel T. Novel antiplatelet agents in cardiovascular disease. J Cardiovasc Pharmacol Ther. 2020;25(3):191–200. https://doi.org/10.1177/1074248419899314.
Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;1–79. https://doi.org/10.1093/eurheartj/ehaa575.
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2018;39:119–77.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15.
Gremmel T, Michelson AD, Frelinger AL, Bhatt DL. Novel aspects of antiplatelet therapy in cardiovascular disease. Res Pract Thromb Haemost. 2018;2:439–49.
Winter M, Schneeweiss T, Cremer R, Biesinger B, Hengstenberg C, Prüller F, et al. Platelet reactivity patterns in patients treated with dual antiplatelet therapy. Eur J Clin Investig. 2019;49:e13102.
Jennings L. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248–57.
Wadowski PP, Pultar J, Weikert C, Eichelberger B, Panzer B, Huber K, et al. Protease-activated receptor-mediated platelet aggregation in acute coronary syndrome patients on potent P2Y 12 inhibitors. Res Pract Thromb Haemost. 2019;3:383–90.
Badr Eslam R, Lang IM, Koppensteiner R, Calatzis A, Panzer S, Gremmel T. Residual platelet activation through protease-activated receptors (PAR)-1 and -4 in patients on P2Y12 inhibitors. Int J Cardiol. 2013;168:403–6.
Gremmel T, Durstberger M, Eichelberger B, Koppensteiner R, Panzer S. Calcium-channel blockers attenuate the antiplatelet effect of clopidogrel. Cardiovasc Ther. 2015;33:264–9.
Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp CW. Calcium-channel blockers decrease clopidogrel-mediated platelet inhibition. Heart. 2010;96:186–9.
Sibbing D, von Beckerath N, Morath T, Stegherr J, Mehilli J, Sarafoff N, et al. Oral anticoagulation with coumarin derivatives and antiplatelet effects of clopidogrel. Eur Heart J. 2010;31:1205–11.
Gilard M, Arnaud B, Cornily J-C, Le Gal G, Lacut K, Le Calvez G, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin. J Am Coll Cardiol. 2008;51:256–60.
Helten C, Mourikis P, Dannenberg L, M’Pembele R, Trojovsky K, Ayhan A, et al. A novel mechanism of ACE inhibition–associated enhanced platelet reactivity: disproof of the ARB-MI paradox? Eur J Clin Pharmacol. 2020;76:1245–51.
Verstraete M. Pharmacotherapeutic aspects of unfractionated and low molecular weight heparins. Drugs. 1990;40:498–530.
Mayer K, Bernlochner I, Braun S, Schulz S, Orban M, Morath T, et al. Aspirin treatment and outcomes after percutaneous coronary intervention. J Am Coll Cardiol. 2014;64:863–71.
Sibbing D, Aradi D, Alexopoulos D, ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–37.
Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–69.
Gremmel T, Frelinger A, Michelson A. Platelet physiology. Semin Thromb Hemost. 2016;42:191–204.
Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension. J Am Coll Cardiol. 2018;71:1474–82.
ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction. Circulation. 1998;97:2202–12.
Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Lancet. 2002;360:752–60.
Pfeffer MA, McMurray JJV, Velazquez EJ, Rouleau J-L, Køber L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906.
Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc. 2016;91:51–60.
Yang JH, Hahn J-Y, Bin SY, Choi S-H, Choi J-H, Lee SH, et al. Angiotensin receptor blocker in patients with ST segment elevation myocardial infarction with preserved left ventricular systolic function: prospective cohort study. BMJ. 2014;349:g6650.
Gremmel T, Kopp CW, Seidinger D, Koppensteiner R, Steiner S, Panzer S. Preserved thrombin-inducible platelet activation in thienopyridine-treated patients. Eur J Clin Investig. 2013;43:689–97.
Gremmel T, Steiner S, Seidinger D, Koppensteiner R, Panzer S, Kopp C. In vivo and protease-activated receptor-1-mediated platelet activation but not response to antiplatelet therapy predict two-year outcomes after peripheral angioplasty with stent implantation. Thromb Haemost. 2014;111:474–82.
Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van De Werf F, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33.
Schieffer B, Bünte C, Witte J, Hoeper K, Böger RH, Schwedhelm E, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44:362–8.
Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schömig A, et al. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53:849–56.
Gremmel T, Panzer S. Clinical, genetic and confounding factors determine the dynamics of the in vitro response/non response to clopidogrel. Thromb Haemost. 2011;106:211–8.
Chen W-H, Lee P-Y, Ng W, Tse H-F, Lau C-P. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol. 2004;43:1122–6.
Al-Azzam SI, Alzoubi KH, Khabour O, Alowidi A, Tawalbeh D. The prevalence and factors associated with aspirin resistance in patients premedicated with aspirin. Acta Cardiol. 2012;67:445–8.
Von Pape KW, Dzijan-Horn M, Bohner J, Spannagl M, Weisser H, Calatzis A. Control of aspirin effect in chronic cardiovascular patients using two whole blood platelet function assays: PFA-100 and multiple electrode aggregometry. 2007;27(3):155–60. https://doi.org/10.1007/978-3-540-36715-4_49.
Bernlochner I, Mayer K, Orban M, Morath T, Jaitner J, Rössner L, et al. Ticagrelor versus prasugrel in patients with high on-clopidogrel treatment platelet reactivity after PCI: the ISAR-ADAPT-PF study. Platelets. 2016;27:796–804.
Wadowski PP, Weikert C, Pultar J, Lee S, Eichelberger B, Koppensteiner R, et al. Ticagrelor inhibits toll-like and protease-activated receptor mediated platelet activation in acute coronary syndromes. Cardiovasc Drugs Ther. 2020;34:53–63.
Funding
Open Access funding provided by Medical University of Vienna. This work was supported by the “Medical Scientific Fund of the Mayor of the City of Vienna,” grant number 14016, and the “Anniversary Fund of the Austrian National Bank,” grant number 16155, to Thomas Gremmel.
Author information
Authors and Affiliations
Contributions
Maximilian Tscharre: writing the manuscript, analysis
Patricia P. Wadowski: data collection, critical revision, and final approval
Constantin Weikert: data collection, critical revision, and final approval
Joseph Pultar: data collection, critical revision, and final approval
Beate Eichelberger: data collection, critical revision, and final approval
Simon Panzer: design, critical revision, and final approval
Thomas Gremmel: design, data collection, analysis, writing the manuscript, critical revision, and final approval
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study protocol was approved by the local Ethics Committee of the Medical University of Vienna and was in accordance with the Declaration of Helsinki and its later amendments.
Consent to Participate
Written informed consent was obtained from all study participants.
Consent for Publication
Written informed consent was obtained from all study participants.
Code Availability
All statistical analyses were generated with Statistical Package for Social Sciences (IBM SPSS version 24, Armonk, New York, USA) and R 3.6.3.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tscharre, M., Wadowski, P.P., Weikert, C. et al. Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in Acute Coronary Syndrome: Implications for Platelet Reactivity?. Cardiovasc Drugs Ther 35, 1183–1190 (2021). https://doi.org/10.1007/s10557-020-07128-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07128-0