Abstract
The recent emergence of the coronavirus disease 19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in China is now a global health emergency. The transmission of SARS-CoV-2 is mainly via human-to-human contact. This virus is expected to be of zoonotic origin and has a high genome identity to that of bat derived SARS-like coronavirus. Various stringent measures have been implemented to lower person-to-person transmission of COVID-19. Particular observations and attempts have been made to reduce transmission in vulnerable populations, including older adults, children, and healthcare providers. This novel CoV enters the cells through the angiotensin-converting enzyme 2 (ACE2) receptor. There is a higher risk of COVID-19 infection among those with preexisting cardiovascular diseases (CVD), and it has been connected with various direct and indirect complications, including myocarditis, acute myocardial injury, venous thromboembolism, and arrhythmias. This article summarizes the various cardiovascular complications and mechanisms responsible for the same with COVID-19 infection. For the benefit of the scientific community and public, the effect of COVID-19 on major vital organs such as the kidneys, liver, and intestines has been briefly discussed. In this review, we also discuss drugs in different stages of clinical trials and their associated complications, as well as the details of vaccines in various stages of development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On March 11, 2020, the World Health Organization (WHO) declared the coronavirus disease a global pandemic. In December 2019, a group of pneumonia cases caused by a newly identified β-coronavirus was reported in Wuhan, China [1, 2]. On January 12, 2020, WHO named this coronavirus as the 2019-novel coronavirus (2019-nCoV). Then WHO officially called the disease coronavirus disease 2019 (COVID-19), and on February 11, 2020, the Coronavirus Study Group (CSG) of the International Committee proposed the name for the virus as SARS-CoV-2 [3]. On January 7, 2020, Chinese scientists isolated SARS-CoV-2 from a patient and determined the genome sequencing [4]. The virus has spread to over 20 countries, with 20,530,324 patients and 746,022 deaths as of August 12, 2020. The clinical observations of disease patterns reveal that infections have a direct impact on the cardiovascular system and, based on this revelation, the worldwide medical fraternities are demanding special care for patients with heart diseases. An earlier history of CVD influences the magnitude of COVID-19 infections, in most cases, and leads to clinical complications [5,6,7]. Case diaries of patients show that the viral infection causes injury to cardiomyocytes, and the reports from Li et al. (2020) point out that at least 8.0% of COVID-19 patients suffered acute myocardial injury [8] (Table 1).
In addition, individuals with preexisting cardiovascular risk factors such as hypertension and diabetes are exhibiting severe health consequences. One report from China reveals that out of 99 cases, cardiac-cerebrovascular diseases were reported for 40% of patients [9, 10]. In patients with cardiovascular and metabolic comorbidities, induced complications make them prone to poor prognosis. Based on these findings, regulatory agencies and policymakers have alerted the public to the consequences of risks associated with these categories of people during infection. Thus, COVID-19 is now a matter of significant concern and attention to both biomedical and clinical research. The principal aim of this review is to reveal the link between CVD and COVID-19 infection and explain the science behind the same. We have also reviewed the impact of COVID-19 on other organs, therapies for control and management, and adverse cardiac effects associated with ongoing treatments.
General Symptoms and Incubation Period of Virus
Coronaviruses are enveloped positive-sense RNA viruses; under an electron microscope, they are revealed to have spike-like projections giving them a crown-like appearance; hence the name coronavirus [11]. The Coronaviridae family (order Nidovirales) has been classified into four genera of CoVs: Alphacoronavirus (alphaCoV), Betacoronavirus (betaCoV), Deltacoronavirus (deltaCoV), and Gammacoronavirus (gammaCoV). Overall, evaluations indicate approximately 5% to 10% of acute respiratory infections are due to these viruses, and 2% of the population are healthy carriers of a CoV [12, 13]. Four coronaviruses can generally cause mild respiratory disease, i.e., HKU1, NL63, 229E, and OC43 have been in circulation among humans [14]. COVID-19 is caused by an RNA virus belonging to the genus Betacoronavirus [15]. The spike glycoprotein of the SARS-CoV-2 virus has two subunits: S1 and S2 (Fig. 1). S1 binds to the cell surface receptors, while S2 fuses with the cell membrane. TMPRSS2, a host transmembrane serine protease helps the virus access the cells by two diverse mechanisms; first, on the cell membrane surface, the spike S1 subunit binds to the ACE2, the ACE2 receptor is cleaved by the activation of the spike by TMPRSS2. Additionally, TMPRSS2 causes an irreversible conformational change by acting on the S2 subunit, leading to the virus fusion to the cell membranes; then it enters the cell [16,17,18].
Transmission occurs primarily via direct person-to-person contact or from an infected individual through droplets spread by coughing or sneezing. After viral exposure, the symptoms of COVID-19 become visible within 2–14 days, which includes fever, dry cough, and shortness of breath [19]. The severe cases showed respiratory, hepatic, gastrointestinal, and cardiovascular complications leading to mortality [20].
COVID-19 and the Cardiovascular System
Novel SARS-CoV-2 has been demonstrated to interact with ACE2, and enter the host’s cells, particularly cardiac myocytes and alveolar epithelial cells [21]. The ACE2 has a broad expression pattern in the human body with a powerful expression observed in the heart, lungs, gastrointestinal system, and kidneys. Additionally, ACE2 plays an essential role in the neurohumoral regulation of the cardiovascular system. The binding of SARS-CoV-2 to ACE2 causes acute myocardial and lung injury through the alternation in ACE2 signaling pathways [22]. ACE2 protects the heart against activation of the renin-angiotensin-aldosterone system (RAAS) because it converts angiotensin II to angiotensin (1–7). Angiotensin II is a vasoconstrictor, proinflammatory mediator, and damages capillary endothelium, while angiotensin (1–7) is a vasodilator. However, the virus entry causes down-regulation of ACE2 and increases angiotensin II levels, leading to increased heart damage. Thus, increased ACE2 receptor density will increase the viral load, but it remains likely to mitigate heart injury [23]. COVID-19 cases are escalating morbidity in patients with cardiovascular problems. Infection affects cardiac relevant biochemical pathways such as the ACE2 signaling pathway, cardiac muscle integrity, fibrinogen pathways, redox homeostasis, and induces a break in plaque associated with the stent, and finally, aggravates a myocardial injury and dysfunction [24].
Hyper Coagulation in COVID-19
COVID-19 patients with a history of diabetes, hypertension, and stroke on ventilators who underwent serological testing, showed the presence of anticardiolipin IgA antibodies and anti β2glycoprotein I IgA and IgG antibodies. These antiphospholipid antibodies abnormally target phospholipid proteins, rarely leading to thrombotic events [25]. Studies have found that some patients have unusual coagulation functions, and almost all critically ill have a coagulation disorder [26, 27]. It is known that acute inflammatory response caused by severe infection or sepsis can affect the coagulation and fibrinolytic system in multiple ways. Additionally, there is a specific correlation between ACE2 and coagulation [28].
COVID-19 infected patients can have a higher risk of venous thromboembolism (VTE) [29]. Increased D-dimer levels (>1 g/L) was often linked with in-hospital death, as reported by a multicenter retrospective cohort study from China [30]. Studies from China pointed out that elevated D-dimer (>0·5 mg/L) was found in 260 (46%) of 560 patients. In another study, approximately 183 patients with a mean D-dimer concentration of 2·12 mg/L (range 0·77–5·27) did not survive and survivors had a concentration of 0·61 mg/L (0·35–1·29) [31, 32]. A small prospective study from Italy showed higher baseline D-dimer levels in 16 patients with ARDS admitted to ICU [33]. Tang et al. (2020) reported an elevated level of D-dimer and fibrin degradation products (FDP) for non-survivors compared to survivors. During the disease condition, approximately 71.4% of non-survivors fit the clinical guidelines for disseminated intravascular coagulation (DIC) [32]. Severely ill patients with long-term immobilization are naturally at higher risk for VTE. In such patients, because of vascular inflammation, endothelial dysfunction and a hypercoagulable state were seen. In some studies, in patients with novel coronavirus pneumonia (NCP), the abnormalities in the coagulation system can be seen, mostly in a hypercoagulable state, which can quickly induce thrombus formation [9, 10]. There may be local embolism in the small vessels and microvessels of the relevant target organs. Some critically ill patients with high D-dimer are expected to have deep vein thrombosis and aortic embolism. This will cause the condition to worsen sharply. Therefore, the abnormal concentration and activity of ACE2 may affect the coagulation system in acutely ill hospitalized patients [28]. For these patients, direct oral anticoagulants and antiviral treatments, unfractionated heparin/low molecular weight heparins, or mechanical prevention, are advised.
Myocardial Infarction and COVID-19
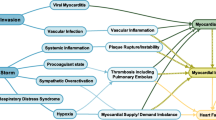
In COVID-19 patients, cardiac damage occurs in many ways. Infection, inflammation, and fever make the blood more prone to clotting and interfere with the body’s ability to dissolve clots. In some patients, even if their arteries do not have fatty, calcified flow-limiting blockages, they may suffer heart damage that imitates heart attack injury, and the situation is known as myocardial infarction type 2 [34]. This can happen when the heart muscle is deprived of oxygen, and oxygen deprivation is one of the clinical symptoms of COVID-19 [35]. The metabolic demand of many organs, including the heart, is increased during fever and inflammation. If the lungs are infected, the stress level is increased, and this will affect the gas exchange, which can further reduce oxygen supply to the heart muscle. Since this virus directly affects the heart, patients with COVID-19 show cardiac muscle inflammation, including among groups who were previously healthy with no cardiac problems. This nature of inflammation leads to cardiac muscle damage, variations in heart rhythm, and disturbs the optimal blood pumping. A case report from Italy points out that even in the absence of lung damage in healthy adults, COVID-19 could affect heart function even after the acute phase is resolved. There are reports that some patients develop life-threatening myocarditis with COVID infection via severe inflammation in the heart muscle [36]. This can happen even in patients with no preexisting risk factors (Fig. 2).
Elevated troponin is an important prognostic marker in COVID-19, even in those without CVD. The highest mortality is in those with CVD and raised troponins, followed by those with raised troponins, but no CVD. Those with CVD, but no raised troponins, have lower mortality. The lowest mortality is in those with neither CVD nor raised troponins. Serial measurements of troponins and N-terminal pro-B-type natriuretic peptide (NT-proBNP) show a rising trend in those who do not survive compared to those who survive. In the latter, the levels remain stationary [37]. Guo et al. (2020) and Shi et al. (2020) present a report on a cohort study of 416 hospitalized patients with COVID-19 who had evidence of myocardial injury manifested by elevation of high-sensitivity troponin I (TnI) levels [37, 38]. A significantly higher in-hospital mortality rate (42 of 82 [51.2%]) was seen in patients with increased TnI levels compared with those without increased TnI (15 of 335 [4.5%]).
Moreover, TnI elevation was associated with higher mortality rates for those with myocardial injury. Their data provide additional novel insights that TnT levels are significantly connected with levels of NT-proBNP and C-reactive protein (CRP), proposing a relation between myocardial stress and inflammation. A report of 150 patients from the fever clinic of Tongji Hospital, Wuhan, showed that the hypersensitive C-reactive protein (hs-CRP) and serum creatinine levels were higher [39]. Patients with myocardial injury also have confirmation of more severe systemic inflammation, including greater leukocyte counts and higher levels of CRP, procalcitonin, and high levels of other biomarkers of myocardial injury and stress, such as elevated creatine kinase, myoglobin, and NT-proBNP [34]. Dr. Bonow and coauthors (2020) noted that patients with chronic coronary artery disease have an increased risk of developing acute coronary syndrome during severe infection. This condition leads to a drastic increase in myocardial demand during infection or critical systemic inflammatory stress that could lead to atherosclerotic plaque instability, rupture, vascular and myocardial inflammation [40]. Systemic inflammation can also result in coronary plaque rupture in CVD patients and cause stent thrombosis [41].
Cytokine Storm and Heart Damage
Multiple studies reported that inflammatory markers are increased with COVID-19 infection, ranging from CRP, ferritin, interleukin-6 (IL-6), interleukin-1β (IL-1β), interferon-γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α) [42,43,44], leading to cytokine storms. Therefore, they can be used as prognostic markers to conclude the severity of COVID-19 infection. The cytokine storm is a complex network of severe molecular events, including a clinical phenotype of systemic inflammation, multi-organ failure, hyper-ferritinemia. It is generated by the activation of an innumerable amount of white blood cells, including B cells, T cells, NK cells, macrophages, dendritic cells, neutrophils, monocytes, and resident tissue cells, such as epithelial and endothelial cells, which release high amounts of proinflammatory cytokines [45]. Several immune pathways and proinflammatory cytokines have been induced by SARS-CoV-2 infection, especially CC chemokine ligand (CCL)2, (CXC chemokine) CXCL2, CCL8, CXCL1, IL33, CCL3L1 in BALF and CXCL10, tumor necrosis factor superfamily TNF Superfamily Member 10 (TNFSF)10, tissue inhibitors of metalloproteinases (TIMP)1, C5, IL18, amphiregulin, neuregulin1, IL10 in PBMC, indicating sustained inflammation and cytokine storm in patients [46]. In a retrospective, multicenter study in Wuhan, China, 150 confirmed COVID-19 cases reported that the mortality was due to virally driven hyper inflammation, which included elevated ferritin and IL-6 [43].
Patients with CVD are at higher risk of cytokine storms. A cytokine storm begins with the activations of cytokine secreting cells with innate and adaptive immune mechanisms. Further, patients who have COVID-19 with myocardial injury have clinical proof of a higher rate of acute respiratory distress syndrome, and they more frequently require assisted ventilation than those without myocardial injury [40]. Myocardial injury may occur through diverse mechanisms, mainly mediated via ACE2 and other proposed mechanisms of cardiac participation comprising cytokine storm, arbitrate among subtypes of T helper cells [30], and severe pneumonia causes hypoxia. This leads to ischemic cardiac tissue, which increases intracellular calcium leading to the apoptosis of cardiac myocyte [47]. This causes a troponin leak and an elevated BNP level. There are reports that in rabbits, coronavirus infection has resulted in acute and even chronic heart failure [48], which could be related to the human strain of coronavirus [49].
Endothelial Dysfunction in COVID-19
Endothelial cell injury plays a vital role in the pathogenesis of multi-organ failure in COVID-19. The endothelium is one of the largest organs in the human body [50]. The endothelial cells express ACE2 receptors, and the viral entry causes major clinical conditions such as high blood pressure [51,52,53], kidney disease [54], cerebrovascular and neurologic disorders [55, 56]. The cardiovascular system is protected by the endothelial cells, and the proteins they release will influence everything from blood clotting to the immune response. Endothelial damage leads to excessive cardiovascular impairment and causes extempore heart attacks in COVID-19. Endothelial cell damage may cause blood vessel inflammation, leading to plaque rupture and heart attack [57, 58]. Due to the devastating immune-inflammatory response and the subsequent cytokine storm, the heart status becomes exacerbated via inflammation-induced heart failure. The factors promoting endothelial dysfunction are discrepancies between reactive oxygen species production and nitric oxide reduction, remodeling of the left ventricle, fibrosis by differentiation of fibroblasts into myofibroblasts following monocytes secretion of transforming growth factor-beta (TGFβ) [59, 60]. In COVID-19 patients with comorbidities, the dysfunctional endothelial response to the infection could also induce activation of the coagulation pathway(s) [61]. Criel et al. (2020) and Bompard et al. (2020) reported the possibility of deep vein thrombosis and acute pulmonary embolism in COVID-19 patients [62, 63]. These data substantiate and hold up a fundamental SARS-CoV-2-related endothelial dysfunction with an augmented risk of venous thromboembolic disease, systemic vasculitis, endothelial cell apoptosis, and inflammation in various organs [64,65,66,67].
Hypertension and COVID-19
It is unclear whether uncontrolled blood pressure is a risk factor for acquiring COVID-19, or whether controlled blood pressure among patients with hypertension is or is not less of a risk factor. The results of pooled analysis data reported by Lippy et al. (2020) pointed out that hypertension might be linked with up to 2.5-fold more significant risk of lethal COVID-19, particularly with older individuals. In COVID-19 illness, through ACE2 receptors, the virus enters the lung, and patients with hypertension have worse outcomes than those with any other underlying condition [68]. In COVID-19 patients, hypertension and other forms of CVD were found frequently, and ACE inhibitors and angiotensin receptor blockers (ARBs) were often used for the treatment, which results in an upregulation of ACE2. There are hypotheses that ACE2-stimulating drugs used to treat hypertension can increase the risk of developing lethal COVID-19. Fang et al. (2020) reported that patients treated with ACE2-elevating drugs for hypertension, diabetes, or cardiac diseases are at increased risk for COVID-19 infection and should, therefore, be monitored for ACE2-modulating medications, such as ACE inhibitors or ARBs [69].
Contrary to this hypothesis, data from various cardiological societies support the continued use of ACE inhibitors or ARBs in patients with hypertension hospitalized with COVID-19 [70]. This strategy had significantly better survival compared with similar hypertensive patients not on these drugs. This hypothesis was derived in observational, propensity score-matched analyses of over 3430 patients hospitalized at various Chinese hospitals during December 2019–February 2020. There are other reports from other affected countries to support this claim, and based on these clinical observations, all clinicians are advised to continue ACE inhibitors and ARB blockers for patients who are already on these drugs for their blood pressure [70]. More research is essential to identify the possible mechanistic association between COVID-19 and CVD outcomes. Lippy and Plebani (2020) [71] pointed out that the meta-analysis study data showed increased blood levels of procalcitonin, a peptide hormone produced by the thyroid gland, lungs, and intestine, are associated with more severe forms of COVID-19. There are reports that low platelet counts are also associated with an increased risk of severe disease and mortality in COVID-19 patients [72].
COVID-19 and Other Organs
ACE2 is not only the access of COVID-19 in the lung but also probably involved in the development of lung injury. Zuo et al. (2020) showed that ACE2 protein is mainly expressed in 1.4% of type II alveolar epithelial cells [73]. Kuba et al. (2020) and Imai et al. (2020) showed that blocking the renin-angiotensin signal pathway can improve severe acute lung injury caused by SARS-CoV spike protein, suggesting that RAAS, composed of ACE, angiotensin II, and type 1a angiotensin II receptor (AT1a), promote the pathogenesis of the disease, induce pulmonary edema, and impair lung function [74, 75]. Hence, ACE2 plays a role in improving severe pulmonary edema and acute lung failure as the RAAS counter regulator.
Genetic analysis by Zhang et al. [76] showed high levels of ACE2 expression in esophageal stratified epithelial cells and ileocolic absorbable epithelial cells, suggesting a potential route of transmission via the gastrointestinal system. Besides fever and cough, the most common infection symptoms are nausea, vomiting, and diarrhea, which is more severe in patients infected with COVID-19 than SARS-CoV and MERS-CoV [9, 77]. COVID-19 entry may be associated with ACE2 abnormal expression and dysfunction in the gastrointestinal tract, leading to intestinal inflammation [28]. ACE2/Ang-(1–7) axis is known as the main peptide on counteracting Ang II effects and reducing inflammation. The intestine is one of the possible target organs for COVID-19 infection, but whether the digestive system is a transmission route requires much further study [78].
COVID-19 can enter renal tubular cells by binding to ACE2 and can lead to cytotoxicity and abnormal renal function. Bile ducts are found to have a high abundance of the neo coronavirus receptor ACE2, while hepatocytes expressed a very low amount [79]. These show that liver damage in COVID-19 may be caused by the virus directly binding to ACE2-positive bile duct cells and causing bile duct dysfunction or toxic side effects caused by therapeutic drugs [80]. Direct entry of the virus to liver cells is not reported anywhere. These results suggest the need for attention to patients’ liver reactions, especially those related to bile duct cell function, and thus the need for special care for patients with neo coronary pneumonia who have an abnormal liver function.
Factors Affecting COVID-19 Infection
Innate Immunity in COVID-19
Innate immunity may be the key to defeat SARS-CoV-2 infection. It serves as the first line of antiviral defense and could participate in a significant role in the progression of the cytokine storm [81]. During infection, the viral genome attachment to the receptors activates the innate immune response signaling pathway. The cells of innate immunity are sentinels to recognize the viral invasion by binding to the coronavirus’s RNA. This leads to type I interferon (IFN-I) expression and other proinflammatory cytokines that defend against viral infection at the access place [82]. In innate immunity, macrophages are very significant, which acquire heterogeneous subsets, including monocyte-derived macrophages and tissue-resident population with an array of distinguishing characteristics from (classically activated macrophage) M1 to (alternatively activated macrophage) M2-like phenotype [83]. Wang et al. (2020) reported that ACE2 receptors in alveolar macrophages lead to the activation and secretion of inflammatory cytokines and monocyte infiltration to lungs after virus binding [84]. In severe conditions, the activation and accumulation of monocytes and macrophages generate abandoned cytokine storms that lead to the modification of the M1 to M2 phenotype of alveolar macrophages. This results in inflammatory injuries and fibrosis of respiratory tracts [84, 85].
Smoking and its Relationship with COVID-19
Smoking increases the risk of severe symptoms developing during COVID-19 infection. Studies stipulate that, compared to nonsmokers, smoker patients may have significantly augmented unfavorable health outcomes regarding COVID-19, in addition to being admitted to intensive care with the need for mechanical ventilation and severe health issues [86, 87]. Smoking is now identified as a risk factor for several other respiratory infections, such as colds, influenza, pneumonia, and tuberculosis [88]. Among patients with severe respiratory diseases, smoking is also related to the progression of acute respiratory distress syndrome, which is a significant problem for extreme cases of COVID-19 [89,90,91]. Various retrospective multicenter cohort studies from China pointed out that smoking causes undesirable effects during COVID-19. A study conducted by Guan et al. (2020) pointed out that among a patient population of 1099, 17% were current smokers and showed very severe symptoms, and over 5% were earlier smokers [31]. There are some studies that show the severe risk associated with smokers during COVID-19 infection compared to nonsmokers [31, 92, 93].
Impact of Gender in COVID-19
It is also observed that men are more prone to COVID-19 complications than women. There are some scientific explanations for this. Sama et al. (2020) reported that in two independent cohorts of patients with cardiac problems, the concentration of ACE2 in plasma is higher in men than in women, neither an ACE inhibitor nor an ARB was associated with higher ACE2 plasma concentrations [94]. The findings might explain the higher incidence and fatality rate of COVID-19 in men. The adverse effect of COVID-19 activity is due to the renin-angiotensin system’s over-activation, and there are reports that the virus can activate a disintegrin and metalloproteases-17 (ADAM-17), which is a crucial regulator of tissue and plasma ACE2 and can cleave tissue ACE2 and increases plasma ACE2, which is more harmful. The presence of an excessive amount of ACE2 in plasma induces cardiac toxicity via renin-angiotensin overactivity. This enzyme also causes a systemic inflammatory response and reduces the cardioprotective effect of ACE2 in tissue. The reduction of ACE2 in tissue amplifies cardiovascular complications significantly, and therefore the inhibition of ADAM-17 is useful to protect COVID-19 patient’s hearts if there is no off-target effect [95, 96]. There are reports that in non-small cell lung cancer cell lines, estradiol increases the expression levels and activity of ADAM-17 [97]. This finding would suggest higher shedding of ACE2 in women and could, at least partially, explain the reduced incidence of COVID-19 in women compared to men [98].
Aging and COVID-19
The susceptibility and severity of COVID-19 in older patients show that age is also a strong risk factor. Aging is associated with depletion of immune power and a weak cardiovascular function. There are reports that older persons with CVD and reduced ACE2 levels will be expected to be more susceptible to the exaggerated inflammation with further reduction in ACE2 expression in the context of COVID-19, exhibiting greater disease severity. Besides this, diabetes and hyperlipidemia, which are the traditional CVD risk factors, can alter the immune functions. This dysregulated immunologic status could raise the risk of CVD occurrence [99,100,101,102]. Thus, CVD may be an indicator of immunologic dysregulation or aging, and it can indirectly relate to the prognosis of COVID-19. Similarly, in patients with hypertension and CVD, elevated expression of ACE2 may increase the vulnerability of SARS-CoV-2 [103].
Drug Therapy in COVID-19
The best strategy for preventing COVID-19 is to take preventive measures. There are no precise therapies approved by WHO, Centers for Disease Control and Prevention (CDC), and the US Food and Drug Administration (FDA) for COVID-19 [104,105,106]. Vaccines and monoclonal antibodies against SARS-CoV-2 are in various stages of development [107]. Several other therapies under investigation are at different lab experiment stages, which include drugs targeting SARS-CoV-2 cell invasion and replication [108]. An in vitro study indicated the efficacy of chloroquine (anti-malarial drug) and hydroxychloroquine (a medicine for rheumatoid arthritis or systemic lupus erythematosus treatment) in blocking SARS-CoV-2 cell entry is probably through affecting endosomal pH and by the glycosylation of ACE2 receptors [109]. The doses of the drugs used in the in vitro study were chloroquine 500 mg twice daily and hydroxychloroquine 400–600 mg twice a day, and trials with these agents are ongoing for patients [110,111,112,113]. Gautret et al. (2020) reported that in most COVID-19 patients, hydroxychloroquine is useful for clearing the viral nasopharyngeal carriage of SARS-CoV-2 within 3 to 6 days [114]. Pfizer has reported data on clinical trials performed in France against COVID-19. They used azithromycin (Zithromax) along with hydroxychloroquine [115]. Geleris et al. (2020) showed that hydroxychloroquine treatment was not associated with the risk of intubation or death. The results of the study should not be taken to preclude either benefit or harm of hydroxychloroquine treatment. In their study, the results do not support hydroxychloroquine at present, without outside randomized clinical trials testing its efficacy [116]. However, there are warnings from other groups on the effectiveness of these combinations in the majority of patients and regarding adverse cardiac effects [117, 118]. Azithromycin, a macrolide antibacterial drug, may prevent bacterial superinfection and have immunomodulatory properties to work as adjunct therapy [114, 119,120,121,122]. The HIV protease inhibitor lopinavir/ritonavir (Kaletra, AbbVie) is also not recommended because of negative clinical trial data and unfavorable pharmacodynamics.
Camostat mesylate (a serine protease inhibitor) approved in Japan for chronic pancreatitis and postoperative reflux esophagitis, among other indications, has been reported to block TMPRSS2 activity and thereby to inhibit SARS-CoV entry into cells [123]. This well-permitted therapy has been proposed as a treatment to prevent SARS-CoV-2 spike protein activation, thus preventing cell entry and controlling the infection. Another drug recommended for treatment is remdesevir. This is a broad-spectrum antiviral that interrupts RNA replication by acting as a nucleotide analog [124] because it is primarily developed to treat Ebola and shown to have in vitro activity against SARS-CoV-2. It prevents the replication ability of MERS-COV in human epithelial cells and mediates entry via human CoV receptors [109, 125]. It is safe in initial trials and is currently undergoing clinical trials in China, Europe, Japan, and the United States [126]. Previous studies report that their use has a clinical advantage in COVID-19 patients with a decrease in the severity of pneumonia and earlier clearance of virus [127]. However, as per regulatory agencies, data is insufficient to suggest for or against the new broad-spectrum antiviral remdesivir.
The antiviral medication oseltamivir, used for the treatment of influenza, has been tried by many patients in China for COVID-19 treatment [77]. Several of the current clinical trials used oseltamivir in the comparison group but not as a proposed therapeutic intervention [128], and this agent has no role in the management of COVID-19 once influenza has been excluded. Umifenovir (arbidol), an antiviral drug mainly for the treatment and prophylaxis of influenza, based on in vitro data suggesting activity against SARS, is currently approved in Russia and China for treatment against COVID-19 [129]. A minimal clinical experience has been described in China with umifenovir for COVID-19 treatment [130]. It is not established, but ongoing randomized clinical trials in China are testing the efficacy of umifenovir for COVID-19.
The combination of HIV protease inhibitor lopinavir/ritonavir was demonstrated to have in vitro activity against SARS-CoV. The drug acts by suppressing coronavirus activity by inhibiting the virus replication, and improved clinical outcomes were reported when used in combination with ribavirin for SARS [131]. There have been reports of its success in treating SARS-CoV-2, though the first randomized control trial did not demonstrate statistically significant benefit among hospitalized patients with COVID-19 [132]. Other proposed strategies include interferon and convalescent serum. IL-6 receptor antagonists used in the treatment of rheumatoid arthritis sarilumab and tocilizumab were also used against COVID-19. For treating acute cytokine release syndrome in patients, chimeric antigen receptor-T cell therapy is used [133]. These may be the possible therapies for COVID-19 patients with markedly elevated IL-6, ferritin, D-dimer, and hs-cTnI level, and those that display elements of cytokine storm or secondary hemophagocytic lymphohistiocytosis. Tocilizumab has been used with reported success for patients with severe COVID-19. There are reports that patients with COVID-19 have elevated levels of acute-phase reactants and inflammatory cytokines leading to cytokine release syndrome (CRS), and tocilizumab is effectively used in in-hospital patients admitted with CRS [134]. Kewan et al. (2020), in their retrospective cohort study, analyzed the resulted favorable outcomes in hypoxic COVID–19 patients who were consecutively admitted between March 13, 2020, and April 19, 2020, and treated with a single intravenous infusion of low–dose tocilizumab. By May, an increasing number of studies had reported the use of tocilizumab in treating COVID-19. A multicenter cohort study by Guaraldi et al. (2020) reported the role of tocilizumab in plummeting the risk of invasive mechanical ventilation/death in a cohort of patients with severe COVID-19 pneumonia with a standard of care treatment. They showed that both intravenous and subcutaneous tocilizumab administration might be capable of reducing the risk of invasive mechanical ventilation or death in patients with severe COVID-19 pneumonia and can be confirmed with randomized clinical trials [135, 136]. Clinical trials with sarilumab just launched in the US [137,138,139,140] and are awaiting results. Monteil et al. [141] showed that clinical-grade human recombinant soluble ACE2 (hrsACE2), which has already been tested in phase 1 and 2 clinical trials [142, 143], can reduce the growth of virus in Vero E6 cells. Additionally, at the early stage of infection, they reported that hrsACE2 could significantly inhibit the infected human blood vessel organoids and kidney organoids. Clinical data are insufficient for the treatment of COVID-19 to suggest either for or against the use of convalescent plasma or hyperimmune immunoglobulin [144]. There are different opinions among various regulatory agencies to recommend immunomodulators such as interferons because of their toxicity and lack of sufficient data on the effectiveness in treating severe acute respiratory distress syndrome and MERS. Similarly, Janus kinase inhibitors, such as baricitinib, are not recommended because of their broad immunosuppressive effects [145].
There are some new data to correlate mortality rate and vitamin deficiency in COVID-19 patients. Medical researchers from Wuhan have recently registered (Identifier: NCT04264533) for a new clinical trial using vitamin C infusion for the treatment of SARS-CoV-2 infected pneumonia cases. In this investigation, intravenous vitamin C or a placebo control at a dose of 24 g/day for 7 days will be tested in 140 patients and will monitor the various needs for mechanical ventilation, organ failure scores and vasopressor drugs, ICU length of stay, and 28-day mortality [146]. Data analysis from ten countries revealed the relation between low vitamin D levels and hyperactive immune systems. Vitamin D strengthens innate immunity and prevents overactive immune responses. This finding could clarify why children are unlikely to die from COVID-19, and an aging population is severely affected by COVID-19. Based on these data, the medical fraternity made suggestions for using vitamin D supplementation to protect against SARS-CoV2 infection [147].
In their guidelines, panel members from the National Institutes for Health, Bethesda, suggested against the regular use of systemic corticosteroids for ventilated COVID-19 patients without ARDS. However, low-dose therapy for adult patients who are undergoing refractory shock is recommended [148]. Consumption of corticosteroids or nonsteroidal anti-inflammatory drugs for other conditions by COVID-19 patients should not be discontinued. Corticosteroids such as dexamethasone (NCT04327401) have broad effects on innate and adaptive immunity [149]. A trial published by the University of Oxford states that this drug cuts deaths in ventilated patients by one third and deaths in other admitted patients receiving oxygen by only one fifth [150, 151]. Although NIH guidelines recommended against the use of angiotensin-converting enzyme inhibitors or ARB for COVID-19 patients, these drugs should not be ceased for CVD patients who are already taking them. Similarly, statins can also be continued for patients with preexisting conditions and not be prescribed for COVID-19 outside of clinical trials. A very recent report suggests that nitric oxide is also being explored for therapeutic use in COVID-19 patients, utilizing its antiviral, antibacterial, and bronchodilation properties [152].
Recently, Cavalli et al. (2020) reported in their retrospective cohort study of patients with COVID-19 and ARDS that a high dose of anakinra, a recombinant interleukin receptor antagonist, was safe with clinical improvement in 72% of patients. This study is registered with ClinicalTrials.gov, NCT04318366, as a part of the COVID-19 Biobank study [153]. Pilkington et al. (2020) reported in their meta-analysis study that favipiravir (avifavir) shows a promising effect against pandemic COVID-19. This drug is an RNA polymerase inhibitor designed to treat influenza and tried for Ebola, among other diseases. However, safety concerns remain: hyperuricemia, teratogenicity, and QTc prolongation have not yet been adequately studied. Favipiravir may be safe and tolerable in short-term use, but more evidence is needed to assess the treatment’s longer-term effects. Given the limitations of the evidence and unresolved safety concerns, caution is warranted in the widespread use of favipiravir [154].
Based on the report regarding the effectiveness of convalescent plasma therapy, many countries have now recommended it. In convalescent plasma therapy, blood plasma from a recovered patient is collected and is transfused to an asymptomatic patient. The first report describing the administration of convalescent plasma to five patients early in the Wuhan’s COVID-19 outbreak was recently published [155]. Salazar et al. (2020) [156], in their case study of 25 patients, pointed out that convalescent plasma administration is a safe treatment option for patients with severe COVID-19 disease.
The anti-inflammatory drug, colchicine, used to treat gout and rheumatic disease has been reported as a promising drug for the treatment of COVID-19. It is an old drug utilized for its anti-inflammatory and antimitotic effects [157]. Deftereos et al. (2020), in their work, reported the effect of colchicine on clinical outcomes in patients with COVID-19. In a randomized multicenter clinical trial, colchicine’s potential was compared with the optimal medical treatment plus colchicine with optimal medical therapy alone (control group) among 105 patients from 16 Greek medical centers. This study concluded with a novel finding that the mechanism of colchicine’s action to treat COVID-19 may be antithrombotic and anti-inflammatory [158].
Drug-Induced Cardiovascular Complications
For the prophylaxis for COVID-19 infection, hydroxychloroquine and azithromycin have been used. Among all, the extensively used antibiotic, azithromycin, is most identified as a rare cause of QT prolongation [159, 160], serious arrhythmias [161, 162], and increased risk for sudden death [163]; higher age and female sex have been indicated as risk factors. This antibiotic can also cause non-pause-dependent polymorphic ventricular tachycardia. Electrophysiologic studies show that both drugs can cause proarrhythmia via mechanisms beyond the blocking of IKr implicated in usual cases of torsade de pointes [164, 165]. On the other hand, safety deliberations for using hydroxychloroquine and azithromycin in medical use have been described by a few authors [166]. For the treatment of COVID-19, there is not enough clinical information to suggest for or against the use of chloroquine or hydroxychloroquine. If used, however, clinicians should monitor patients for adverse effects, especially prolonged QT intervals.
Vaccines at Various Stages of Development
The human clinical testing for the first COVID-19 vaccine launched with unusual rapidity was on March 16, 2020. Approximately, 115 vaccine candidates, of which 78 are confirmed as active, and 37 are unconfirmed (development status cannot be determined from publicly available or proprietary information sources) as of April 8, 2020. Among the 78 confirmed active projects, 73 are currently in preclinical stages [167]. The vaccines recently under clinical trials include mRNA-1273 from Moderna, Ad5-nCoV from CanSino Biologicals, INO-4800 from Inovio, LV-SMENP-DC and pathogen-specific aAPC from Shenzhen Geno-Immune Medical Institute, Covax-19™ from GeneCure Biotechnologies, ChAdOx1 nCoV-19 and MenACWY from the University of Oxford, Covaxin from Bharat Biotech, and ZyCOV from Zydas Cadila Healthcare [168,169,170,171,172,173,174,175,176]. The summary of details of stages of various vaccines is given in Table 2.
Conclusion
COVID-19 is a global pandemic and is a significant health threat worldwide. There is an increase in morbidity and mortality in patients suffering from both cardiovascular complications and COVID-19. Therefore, more considerable attention is needed for viral infection related heart damage at the time of treatment. The cardiologist community has to play an essential role in managing and treating patients affected by this disease. Notably, better awareness of the link between the ACE2 protein, hypertension, and COVID-19 will be valuable for patients with both COVID-19 and CVD. The possibility of testing troponin levels in suspected communities as a primary screening protocol should be considered owing to the low cost and short time consumption. Some promising treatments are under investigation; however, none with proven clinical efficacy has been reported to date.
References
Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27:taaa008.
Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–2.
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74.
Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1:274–81.
Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–53.
Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–8.
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–8.
Chen N, Zhou M, Dong X, Jieming Q, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan China: a descriptive study. Lancet. 2020;395:507–13.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Richman DD, Whitley RJ, Hayden FG. Clinical virology. 4th ed. Washington: ASM Press; 2016.
Chan JF, To KK, Tse H, Jin DY, Yuen KY. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21:544–55.
Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–23.
Liu DX, Liang JQ, Fung TS. Human coronavirus-229E, -OC43, -NL63, and -HKU1. Reference Module in Life Sciences. 2020;B978-0-12-809633-8.21501-X.
Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect. 2020;S1684–1182(20):30082–7.
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.
Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106:5871–6.
Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84:12658–64.
Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9(5):1417.
World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations. Accessed 29 Mar 2020.
Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Napoli RD. Features, evaluation, and treatment coronavirus (COVID-19), in StatPearls [internet]. Treasure Island: StatPearls; 2020. https://www.ncbi.nlm.nih.gov/books/NBK554776/. Accessed 10 Aug 2020.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4.
Groß S, Jahn C, Cushman S, Bär C, Thum T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: from basic science to clinical implications. J Mol Cell Cardiol. 2020;144:47–53.
Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–50.
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38.
Kowalewski M, Fina D, Słomka A, Raffa GM, Martucci G, et al. COVID-19 and ECMO: the interplay between coagulation and inflammation—a narrative review. Crit Care. 2020;24(1):205.
Song J, Wang G, Zhang W, Zhang Y, Li WQ, et al. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7(1):19.
Li SR, Tang ZJ, Li ZH, Liu X. Searching therapeutic strategy of new coronavirus pneumonia from angiotensin-converting enzyme 2: the target of COVID-19 and SARS-CoV. Eur J Clin Microbiol Infect Dis. 2020;39:1021–6.
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;S0:735–1097(20)35008-7.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.
Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7.
Ranucci M, Ballotta A, Di Dedda U. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–51.
Böhm M, Frey N, Giannitsis E, Sliwa K, Zeiher AM. Coronavirus disease 2019 (COVID-19) and its implications for cardiovascular care: expert document from the German Cardiac Society and the World Heart Federation. Clin Res Cardiol. 2020;27:1–14.
Nan J, Jin YB, Myo Y, Zhang G. Hypoxia in acute cardiac injury of coronavirus disease 2019: lesson learned from pathological studies. J Geriatr Cardiol. 2020;17(4):221–3.
Orrico J. Coronavirus and heart. 2020. https://news.harvard.edu/gazette/story/2020/04/covid-19s-consequences-for-the-heart/. Accessed 14 Apr 2020.
Guo T, Fan Y, Chen M, Wu X, Zhang L, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):1–8.
Shi S, Qin M, Shen B, Cai Y, Liu T, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810.
Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. 2020;48(7):567–571.
Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020.
Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798–1800.
Colafrancesco S, Alessandri C, Conti F, Priori R. COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;19(7)102573.
Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8.
Wu C, Chen X, Cai Y, Xia J, Zhou X, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11.
Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69(6):1135–43.
Azkur AK, Akdis M, Azkur D, Sokolowska M, Vande veen W, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–81.
Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60.
Alexander LK, Small JD, Edwards S, Baric RS. An experimental model for dilated cardiomyopathy after rabbit coronavirus infection. J Infect Dis. 1992;166:978–85.
Small JD, Aurelian L, Squire RA, Strandberg JD, Melby EC Jr, Turner TB, et al. Rabbit cardiomyopathy associated with a virus antigenically related to human coronavirus strain 229E. Am J Pathol. 1979;95:709–29.
Cooke JP. The endothelium: a new target for therapy. Vasc Med. 2000;5(1):49–53.
Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:353.
Schiffrin EL, Flack J, Ito S, Muntner P, Webb C. Hypertension and COVID-19. Am J Hypertens. 2020;33:33–373.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9.
Durvasula R, Wellington T, McNamara E, Watnick S. COVID-19 and kidney failure in the acute care setting: our experience from Seattle. Am J Kidney Dis. 2020;76(1):4–6.
Aggarwal G, Lippi G, Michael HB. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020;15(4):385–9.
Mao L, Jin H, Wang M, Hu Y, Chen S, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9.
Froldi G, Dorigo P. Endothelial dysfunction in coronavirus disease 2019 (COVID-19): gender and age influences. Med Hypotheses. 2020;144:110015.
Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130.
Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–94.
Riehle C, Bauersachs J. Key inflammatory mechanisms underlying heart failure. Herz. 2019;44:96–106.
Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67.
Criel M, Falter M, Jaeken J, Van Kerrebroeck M, Lefere I, Meylaerts L, et al. Venous thromboembolism in SARS-CoV-2 patients: only a problem in ventilated ICU patients, or is there more to it? Eur Respir J. 2020;56(1):2001201.
Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56(1):2001365.
Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, Ferraz da Silva LF, Pierre de Oliveira E, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18(6):1517–9.
Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858.
Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–6.
Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. 2020;2(9):1379–82.
Lippi G, Wong J, Henry BM. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–9.
Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21.
Zhang P, Zhu L, Cai J, Fang L, Qin JJ, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681.
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1.
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8.
Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–92.
Kuba K, Imai Y, Rao S, Gao H, Guo F, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–9.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–6.
Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020.
Wang D, Hu B, Hu C, Zhu F, Liu X, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9.
Ding Y, He L, Zhang Q, Huang Z, Che X, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–30.
Chai X, Hu L, Zhang Y, Han W, Lu Z, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020.
Wu J, Song S, Cao HC, Li LJ. Liver diseases in COVID-19: etiology, treatment and prognosis. World J Gastroenterol. 2020;26(19):2286–93.
Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32.
Hajivalili M, Hosseini M, Haji-Fatahaliha M. Gaining insights on immune responses to the novel coronavirus, COVID-19 and therapeutic challenges. Life Sci. 2020;257:118058.
Evren E, Ringqvist E, Willinger T. Origin and ontogeny of lung macrophages: from mice to humans. Immunology. 2020;160(2):126–38.
Wang C, Xie J, Zhao L, Fei X, Zhang H, et al. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Immunol Pathol. 2020.
Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14(2):81–93.
Vardavas CI, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20.
Liu W, Tao Z, Lei W, Ming-Li Y, Kui L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–8.
US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The health consequences of smoking: 50 years of progress—A report by the Surgeon General, Atlanta, 2014. https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm.
World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim Guidance, 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed 13 Mar 2020.
Hsieh S, Zhuo H, Benowitz N, Thompson B, Liu K, et al. Prevalence and impact of active and passive cigarette smoking in acute respiratory distress syndrome. Crit Care Med. 2014;42:2058–68.
Calfee C, Matthay M, Kangelaris K, Siew E, Janz D, et al. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med. 2015;43:1790–7.
Shi Y, Yu X, Zhao H, Wanh H, Zhao R, et al. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108.
Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: A meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656.
Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J. 2020;41:1810–7.
Ingraham NE, Barakat AG, Reilkoff R, Bezdicek T, Schacker T, et al. Understanding the renin-angiotensin-aldosterone-SARS-CoV-Axis: a comprehensive review. Eur Respir J. 2020;56(1):2000912.
Palau V, Riera M, Soler MJ. ADAM17 inhibition may exert a protective effect on COVID-19. Nephrol Dial Transpl. 2020;35(6):1071–1072.
Ren J, Nie Y, Lv M, Shen S, Tang R, et al. Estrogen upregulates MICA/B expression in human nonsmall cell lung cancer through the regulation of ADAM17. Cell Mol Immunol. 2015;12:768–76.
Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol. 2020;115(3):31.
Zidar DA, Al-Kindi SG, Liu Y, Krieger NI, Perzynski AT, Osnard M, et al. Association of lymphopenia with risk of mortality among adults in the US general population. JAMA Netw Open. 2019;2:e1916526.
Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on Atherothrombosis, Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38.
Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16.
Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4.
Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–71.
World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. 2020. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 28 Jan 2020.
CDC Website. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
FDA Website. 2020. https://www.fda.gov/emergency-.
Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–9.
Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141(20):1648–1655.
Wang M, Cao R, Zhang L, Yang X, Liu J, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71.
ClinicalTrials.gov. Comparison of lopinavir/ritonavir or hydroxychloroquine in Patients with mild coronavirus disease (COVID-19). Identifier: NCT04307693. Updated March 13, 2020. https://clinicaltrials.gov/ct2/show/NCT04307693.
ClinicalTrials.gov. Efficacy and safety of hydroxychloroquine for treatment of pneumonia caused by 2019-nCoV ( HC-nCoV ). Identifier: NCT04261517. Updated March 4, 2020. https://clinicaltrials.gov/ct2/show/NCT04261517.
ClinicalTrials.gov. Post-exposure Prophylaxis/ Preemptive Therapy for SARS-Coronavirus-2. Identifier: NCT04308668. Updated March 19, 2020. https://clinicaltrials.gov/ct2/show/NCT04308668.
ClinicalTrials.gov. Chloroquine/hydroxychloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting (COPCOV). Identifier: NCT04303507. Updated March 11, 2020. https://clinicaltrials.gov/ct2/show/NCT04303507
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
Pfizer reports safety data of azithromycin in Covid-19 trial, 2020. https://www.clinicaltrialsarena.com/news/pfizer-data-azithromycin-covid-19-trial/. Accessed 26 Mar 2020.
Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–8.
Fihn SD, Perencevich E, Bradley SM. Caution needed on the use of chloroquine and hydroxychloroquine for coronavirus disease 2019. JAMA Netw Open. 2020;3:e209035.
EMA warns against malaria drugs’ side effects in COVID-19 use. 2020. reuters.com/article/health-coronavirus-ema/ema-warns-against-malaria-drugs-side-effects-in-covid-19-use-idINKCN225219. Accessed 23 Apr 2020.
Amsden GW. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55:10–21.
Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11:90.
Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615.
Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68:479–503.
Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–45.
Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–9.
de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117:6771–6.
ClinicalTrials.gov website. 2020. https://www.clinicaltrials.gov/ct2/results?cond=Coronavirus&term=&type=&rslt=&age_v=&gndr=&intr=remdesivir&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=.
Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–3.
ClinicalTrials.gov. Hydroxychloroquine, oseltamivir and azithromycin for the treatment of COVID-19 infection: An RCT (PROTECT). Updated on July 15, 2020. https://clinicaltrials.gov/ct2/show/NCT04338698.
Khamitov RA, Loginova SIa, Shchukina VN, Borisevich SV, Maksimov VA, Shuster AM. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures [in Russian]. Vopr Virusol. 2008;53:9–13.
Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769–777.
Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–6.
Cao B, Wang Y, Wen D, Liu W, Wang J, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–99.
Xu X, Han M, Li T, Sun W, Wang D, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–10975.
Mastroianni A, Grecoa S, Apuzzo G, De Santisa S, Oriolo C, et al. Subcutaneous tocilizumab treatment in patients with severe COVID-19related cytokine release syndrome: an observational cohort study. EClinicalMedicine. 2020;24:100410.
Kewan T, Covuta F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, et al. Tocilizumab for treatment of patients with severe COVID19: a retrospective cohort study. EClinicalMedicine. 2020;24:100418.
Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:E474–84.
ClinicalTrials.gov. Tocilizumab in COVID-19 pneumonia (TOCIVID-19) (TOCIVID19). Identifier: NCT04317092. Updated March 20; 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092.
ClinicalTrials.gov. Tocilizumab vs CRRT in management of cytokine release syndrome (CRS) in COVID-19 (TACOS). Identifier: NCT04306705. Updated March 17, 2020. https://clinicaltrials.gov/ct2/show/NCT04306705.
ClinicalTrials.gov. Tocilizumab for SARS-CoV2 severe pneumonitis. Identifier: NCT04315480. Updated March 19, 2020. https://clinicaltrials.gov/ct2/show/NCT04315480.
ClinicalTrials.gov. Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19. Identifier: NCT04315298. Updated March 19, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04315298.
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–13.
Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, et al. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52:783–92.
Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234.
NIH. Therapeutic options for COVID-19 currently under investigation, COVID-19 treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/.
Praveen D, Puvvada RC, Aanandhi MV. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int J Antimicrob Agents. 2020;55(5):105967.
Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24(1):133.
Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Research Square 2020;32(7):1195–1198.
Swift D. NIH panel issues guidelines for COVID-19 treatment. 2020. https://www.medscape.com/viewarticle/929203#vp_2. Accessed 22 Apr 2020.
ClinicalTrials.gov. COVID-19-associated ARDS treated with dexamethasone: alliance Covid-19 Brasil III (CoDEX). 2020. https://clinicaltrials.gov/ct2/show/NCT04327401. Accessed 7 Aug 2020.
Mahase E. Covid-19: demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ. 2020;369:m2512.
Johnson RM, Vinetz JM. Dexamethasone in the management of covid-19. BMJ. 2020;370:m2648.
Zamanian RT, Jr Pollack CV, Gentile MA, Rashid M, Fox JC, et al. Outpatient inhaled nitric oxide in a patient with vasoreactive IPAH and COVID-19 infection. Am J Respir Crit Care Med. 2020;202(1):130–132.
Cavalli G, Luca GD, Campochiaro C, Della-Torre E, Ripa M, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020; https://doi.org/10.1016/S2665-9913(20)30127-2
Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir—a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6:45–51.
Shen C, Wang Z, Zhao F, Yang Y, Li J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589.
Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, et al. Treatment of COVID-19 patients with convalescent plasma. Am J Pathol. 2020;190(8):1680–1690.
Rabbani AB, Parikh RV, Rafique AM. Colchicine for the treatment of myocardial injury in patients with coronavirus disease 2019 (COVID-19)—an old drug with new life? JAMA Netw Open. 2020;3(6):e2013556.
Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136.
Choi Y, Lim HS, Chung D, Choi JG, Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed Res Int. 2018;1574806. https://doi.org/10.1155/2018/1574806
Sears SP, Getz TW, Austin CO, Palmer WC, Boyd EA, Stancampiano FF. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcome. 2016;3:99–105.
Huang BH, Wu CH, Hsia CP, Yin CC. Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol. 2007;30:1579–82.
Kezerashvili A, Khattak H, Barsky A, Nazari R, Fisher JD. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J Interv Card Electrophysiol. 2007;18:243–6.
Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–90.
Zhang M, Xie M, Li S, Gao Y, Xue S, et al. Electrophysiologic studies on the risks and potential mechanism underlying the proarrhythmic nature of azithromycin. Cardiovasc Toxicol. 2017;17:434–40.
Capel RA, Herring N, Kalla M, Yavari A, Mirams GR, et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: novel electrophysiological insights and therapeutic potential. Heart Rhythm. 2015;12:2186–94.
Simpson TF, Kovacs RJ, Stecker EC. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19, Published online March 29, 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventriculararrhythmia-risk-due-to-hydroxychloroquine azithromycin-treatment-for-covid-19. Accessed 2 April 2020.
Thanh Le T, Andreadakis Z, Kumar A, Roman RG, Tollefsen S, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–6.
ClinicalTrials.gov. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19). Updated on July 17, 2020, https://clinicaltrials.gov/ct2/show/NCT04283461.
ClinicalTrials.gov. Phase I Clinical trial of a COVID-19 vaccine in 18-60 healthy adults (CTCOVID-19). Updated on May 19, 2020, https://clinicaltrials.gov/ct2/show/NCT04313127.
ClinicalTrials.gov. Safety, tolerability and immunogenicity of INO-4800 for COVID-19 in healthy volunteers. Updated on July 7, 2020. https://clinicaltrials.gov/ct2/show/NCT04336410.
ClinicalTrials.gov. Immunity and safety of Covid-19 synthetic minigene vaccine. Updated on March 19, 2020. https://clinicaltrials.gov/ct2/show/NCT04276896.
ClinicalTrials.gov. Safety and immunity of Covid-19 aAPC vaccine. Updated on March 9, 2020. https://clinicaltrials.gov/ct2/show/NCT04299724.
ClinicalTrials.gov. Therapeutic vaccine trial of COVID-19 for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Updated on June 11, 2020. https://clinicaltrials.gov/ct2/show/NCT04428073.
ClinicalTrials.gov. A study of a candidate COVID-19 vaccine (COV001). Updated on July 8, 2020. https://clinicaltrials.gov/ct2/show/NCT04324606.
COVAXINTM—India’s first indigenous COVID-19 vaccine. https://www.bharatbiotech.com/covaxin.html.
Zydus Cadila’s Covid-19 vaccine enters phase II testing. Updated on August 6, 2020. https://www.clinicaltrialsarena.com/news/zydus-covid-vaccine-phaseii/.
Acknowledgments
The first author thanks the HRD Scheme of the Department of Health Research (DHR), Government of India, Woman Scientist Fellowship, Project Grant No.12013/20/2020-HR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soumya, R.S., Unni, T.G. & Raghu, K.G. Impact of COVID-19 on the Cardiovascular System: A Review of Available Reports. Cardiovasc Drugs Ther 35, 411–425 (2021). https://doi.org/10.1007/s10557-020-07073-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-07073-y