Abstract
Introduction
Warfarin, a racemic mixture of S- and R-enantiomers, is the cornerstone of therapy in patients following cardiac valve replacement. S-warfarin is metabolized to 7-S-hydroxywarfarin by the cytochrome P450 isoform 2C9 encoded by CYP2C9 gene. R-warfarin is metabolized by multiple cytochromes P450. We sought to assess the impact of clinical and genetic factors on circulating warfarin metabolites following valve implantation.
Material and Methods
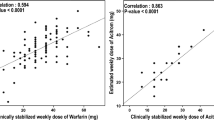
Venous blood was collected from 120 patients after 3 months since elective mitral and/or aortic valve replacement. Plasma S-warfarin, R-warfarin, S-7-hydroxywarfarin, and R-7-hydroxywarfarin were determined using high-performance liquid chromatography. The S-7-hydroxywarfarin/S-warfarin and S-warfarin/R-warfarin (S/R) ratios, along with warfarin sensitivity index (WSI), defined as INR/S-warfarin ratio, were calculated. Vitamin K epoxide reductase complex subunit 1 (VKORC1) c.-1639A, CYP2C9*3 and CYP2C9*2 alleles were determined using real-time polymerase chain reaction.
Results
The S-warfarin was higher in former smokers (p = 0.047) and the VKORC1 c.-1639A allele carriers (p < 0.0001). The S-7-hydroxywarfarin was lower in carriers of the VKORC1 c.-1639A allele (p = 0.0005) and CYP2C9*3 (p = 0.047). The S-7-hydroxywarfarin/S-warfarin ratio was lower in the carriers of CYP2C9*3 (p = 0.008), but not in those with VKORC1 –c.1639A allele. The S/R ratio was higher in patients with hypertension (p = 0.01). The independent predictors of elevated S/R ratio defined as the upper quartile were diabetes (p = 0.045), CYP2C9*3 (p < 0.0001) and CYP2C9*2 (p = 0.0002). The independent predictors of elevated WSI were current smoking (p = 0.049), implantation of mechanical valve (p = 0.006) and VKORC1c.-1639A allele (p = 0.007).
Conclusion
We conclude that not only genetic, but also several clinical factors affect warfarin metabolites in patients following cardiac valve implantation.



Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass graft
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- CV:
-
Coefficients of variability
- CYP2C9 :
-
Cytochrome P450 isoform 2C9 gene
- eGFR:
-
Estimated glomerular filtration rate
- HPLC-UV:
-
High performance liquid chromatography with ultraviolet detection
- INR:
-
International normalized ratio
- SNP:
-
Single nucleotide polymorphism
- S/R ratio:
-
S-warfarin/R-warfarin ratio
- TTR:
-
Time in therapeutic range
- Vd:
-
Volume of distribution
- VKORC1 :
-
Vitamin K epoxide reductase complex subunit 1 gene
- WSI:
-
Warfarin sensitivity index
References
Vahanian A, Alfieri O, Andreotti F, et al. Joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–96.
O’Reilly RA. Studies on the optical enantiomorphs of warfarin in man. Clin Pharmacol Ther. 1974;16:348–54.
Golan DE, Tashjian Jr AH, Armstrong EJ, Armstrong AW. Principles of pharmacology: the pathophysiologic basis of drug therapy, vol. 41. 3rd ed. Philadelphia: Lippincott Williams&Wilkins; 2012.
Takahashi H, Echizen H. Pharmacogenetics of warfarin. Elimination and its clinical implications. Clin Pharmacokinet. 2001;40:587–603.
Chan E, McLachlan AJ, Pegg M, MacKay AD, Cole RB, Rowland M. Disposition of warfarin enantiomers and metabolites in patients during multiple dosing with rac-warfarin. Br J Clin Pharmacol. 1994;37:563–9.
Herman D, Locatelli I, Grabnar I, et al. Influence of CYP2C9 polymorphisms, demographic factors and concomitant drug therapy on warfarin metabolism and maintenance dose. Pharmacogenomics J. 2005;5:193–202.
Miura M, Okuyama S, Kato S, et al. Simultaneous determination of warfarin and 7-hydroxywarfarin enantiomers by high-performance liquid chromatography with ultraviolet detection. Ther Drug Monit. 2011;33:108–14.
Scordo MG, Pengo V, Spina E, Dahl ML, Gusella M, Padrini R. Influence of cytochrome P450 CYP2C9 and 2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther. 2002;72:702–10.
Shikata E, Ieiri I, Ishiguro S, et al. Association of pharmacokinetic (CYP2C9) and pharmacodynamic (factors II, VII, IX, and X; proteins S and C; and gamma-glutamylcarboxylase) genes with warfarin sensitivity. Blood. 2004;103:2630–5.
Franco V, Polanczyk CA, Clausell N, Rohde LE. Role of dietary vitamin K intake in chronic oral anticoagulation: prospective evidence from observational and randomized protocols. Am J Med. 2004;116:651–6.
Takahashi H, Wilkinson GR, Nutescu EA, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese. Caucasians and African-Americans. Pharmacogenet Genomics. 2006;16:101–10.
Meijer K, Kim YK, Schulman S. Decreasing warfarin sensitivity during the first three months after heart valve surgery: implications for dosing. Thromb Res. 2010;125:224–9.
Rost S, Fregin A, Ivaskevicius V, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–41.
Geisen C, Watzka M, Sittinger K, et al. VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb Haemost. 2005;94:773–9.
D’Andrea G, D’Ambrosio RL, Di Perna P, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–9.
Stepien E, Branicka A, Ciesla-Dul M, Undas A. A vitamin K epoxide reductase-oxidase complex gene polymorphism (−1639G>A) and interindividual variability in the dose-effect of vitamin K antagonists. J Appl Genet. 2009;50:399–403.
Zhu Y, Shennan M, Reynolds KK, et al. Estimation of warfarin maintenance dose based on VKORC1 (−1639G-A) and CYP2C9 genotypes. Chemistry. 2007;53:1199–205.
Rettie AE, Tai G. The pharmocogenomics of warfarin: closing in on personalized medicine. Mol Interv. 2006;6:223–7.
Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84:326–31.
Rosendaal FR, Cannegieter SC, Van deer Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9.
Linder MW, Homme MB, Reynolds KK, et al. Interactive modeling for ongoing utility of pharmacogenetic diagnostic testing: application for warfarin therapy. Clin Chem. 2009;55:1861–68.
Liu Y, Jeong H, Takahashi H, et al. Decreased warfarin clearance associated with the CYP2C9 R150H (*8) polymorphism. Clin Pharmacol Ther. 2012;91:660–5.
Lubetsky A, Dekel-Stern E, Chetrit A, Lubin F, Halkin H. Vitamin K intake and sensitivity to warfarin in patients consuming regular diets. Thromb Haemost. 1999;81:396–9.
Giansante C, Fiotti N, Altamura N, et al. Oral anticoagulation and VKORC1 polymorphism in patients with a mechanical heart prosthesis: a 6-year follow-up. J Thromb Thrombolysis. 2012;34:506–12.
Rahman M, BinEsmael TM, Payne N, Butchart EG. Increased sensitivity to warfarin after heart valve replacement. Ann Pharmacother. 2006;40:397–401.
Goldsmith I, Turpie AG, Lip GY. Valvular heart disease and prosthetic heart valves. BMJ. 2002;325:1228–31.
Hamberg AK, Dahl ML, Barban M, et al. A PK–PD model for predicting the impact of age, CYP2C9, and VKORC1 genotype on individualization of warfarin therapy. Clin Pharmacol Ther. 2007;81:529–38.
Lee VW, You JH, Lee KK, Chau TS, Waye MM, Cheng G. Factors affecting the maintenance stable warfarin dosage in Hong Kong Chinese patients. J Thromb Thrombolysis. 2005;20:33–8.
Holstein A, Plaschke A, Ptak M, et al. Association between CYP2C9 slow metabolizer genotypes and severe hypoglycaemia on medication with sulphonylurea hypoglycaemic agents. Br J Clin Pharmacol. 2005;60:103–6.
Hallberg P, Karlsson J, Kurland L, et al. The CYP2C9 genotype predicts the blood pressure response to irbesartan: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs. Atenolol (SILVHIA) trial. J Hypertens. 2002;20:2089–93.
Kornej J, Potpara T, Lip GY. Anticoagulation management in nonvalvular atrial fibrillation: current and future directions. Pol Arch Med Wewn. 2013;123:623–34.
Compliance with Ethical Standards
Conflict of Interest
The authors stated that there are no conflicts of interest regarding the publication of this article.
Research Funding
This study was supported by the grant from National Centre of Science (N N403152340, to A.U.).
Research Involving Human Participants
The study was approved by the University Bioethical Committee according to Declaration of Helsinki.
Informed Consent
All patients gave written informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bryk, A., Wypasek, E., Awsiuk, M. et al. Warfarin Metabolites in Patients Following Cardiac Valve Implantation: A Contribution of Clinical and Genetic Factors. Cardiovasc Drugs Ther 29, 257–264 (2015). https://doi.org/10.1007/s10557-015-6591-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-015-6591-8