Abstract
Purpose
Vitamin K antagonists (VKAs), such as warfarin and acenocoumarol, exert their anti-coagulant effect by inhibiting the subunit 1 of vitamin K epoxide reductase complex (VKORC1). CYP2C9 is a hepatic drug-metabolizing enzyme in the CYP450 superfamily and is the primary metabolizing enzyme of warfarin. Three single nucleotide polymorphisms, two in the CYP2C9 gene, namely CYP2C9*2 and CYP2C9*3, and one in the VKORC1 gene (c.− 1639G > A, rs9923231), have been identified to reduce VKA metabolism and enhance their anti-coagulation effect. The purpose of this study is to evaluate the prevalence of CYP2C9 and VKORC1 polymorphism in Indians receiving VKA-based anti-coagulation after valve surgery and to evaluate the usefulness of genetic information in managing VKA-based anti-coagulation.
Methods
In the current prospective observational study, 150 patients who underwent heart valve surgery and had stable INR were genotyped for VKORC1 (− 1639 G > A), CYP2C9*2, and CYP2C9*3. The VKA dosage was estimated from published algorithms and compared to the clinically stabilized dosage.
Results
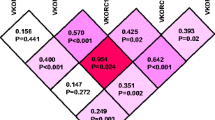
Out of 150 patients, 101 (67.33%) were on warfarin and 49 (32.66%) were on acenocoumarol. Majority of the patients, the 83 in warfarin group and the 40 in acenocoumarol group, had a wild CYP2C9 diplotype. The rest had a mutant (CYP2C9*2 or CYP2C9*3) diplotype. Similarly, 67 patients in the warfarin group and 35 patients in the acenocoumarol group had wild type (G/G) of VKORC1 genotype. The rest had a mutant (G/A or A/A) VKORC1 genotype. In the warfarin group, based on the genotype, 51.5% of the patients were extensive or normal metabolizers, and 47.4% of the patients were intermediate metabolizers of VKAs. In the acenocoumarol group, 61.2% of the patients were extensive or normal metabolizers, and 38.8% of the patients were intermediate metabolizers. Individually, alleles of VKORC1 (− 1639 G > A), CYP2C9*2, and CYP2C9*3 had mean dosage reduction effect on VKA dosage, which co-related to the clinically stabilized dosages (P < 0.0001). Among the VKORC1 (− 1639 G > A) cohort, the reduction in warfarin mean weekly dosage was 13.48 mg as compared to the wild-type category (P < 0.0001) and similarly, the reduction in the mean weekly acenocoumarol dose was 6.07 mg (P < 0.03) as compared to the wild type after adjusting for age, gender, and body mass index.
Conclusion
Single nucleotide polymorphism in the CYP2C9 gene and in the VKORC1 gene is present in nearly 40% of Indian patients. VKORC1 (− 1639 G > A), CYP2C9*2, and CYP2C9*3 genotypes have significant dosage-lowering effects on VKA-based anti-coagulation therapy. The trend in estimated dosages of VKAs co-related to that of observed the clinically stabilized dosage in the cohort. The pharmacogenomic calculators used in this study tend to overestimate the VKA dosages as compared to clinical dosage due to the limitations in the algorithms and in our study. A new algorithm based on a larger dataset capturing the vast genetic variability across the Indian population and relevant clinical factors could provide better results.

Similar content being viewed by others
References
Aquilante CL, Langaee TY, Lopez LM, et al. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin Pharmacol Ther. 2006;79:291–302.
Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12:251–63.
Lenzini P, Wadelius M, Kimmel S, et al. Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin Pharmacol Ther. 2010;87:572–8.
Limdi NA, Wadelius M, Cavallari L, et al. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across three racial groups. Blood. 2010;115:3827–34.
Roth JA, Boudreau D, Fujii MM, et al. Genetic risk factors for major bleeding in patients treated with warfarin in a community setting. Clin Pharmacol Ther. 2014;95:636–43.
Gage BF, Lesko LJ. Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J Thromb Thrombolysis. 2008;25:45–51.
Bristol-Meyers Squibb Company, Coumadin® tablets (warfarin sodium tablets, USP) crystalline; Coumadin® for injection (warfarin sodium for injection, USP). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009218s107lbl.pdf
Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther. 2011;90:625–9.
Lin M, Yu L, Qiu H, Wang Q, Zhang J, Song H. Verification of five pharmacogenomicsbased warfarin administration models. Indian J Pharmacol. 2016;48:258–63.
Shin J, Cao D. Comparison of warfarin pharmacogenetic dosing algorithms in a racially diverse large cohort. Pharmacogenomics. 2011;12:125–34.
Tan GM, Wu E, Lam YY, Yan BP. Role of warfarin pharmacogenetic testing in clinical practice. Pharmacogenomics. 2010;11:439–48.
Moreau C, Pautas E, Gouin-Thibault I, et al. Predicting the warfarin maintenance dose in elderly inpatients at treatment initiation: accuracy of dosing algorithms incorporating or not VKORC1/CYP2C9 genotypes. J Thromb Haemost. 2011;9:711–8.
Shaw PB, Donovan JL, Tran MT, Lemon SC, Burgwinkle P, Gore J. Accuracy assessment of pharmacogenetically predictive warfarin dosing algorithms in patients of an academic medical center anticoagulation clinic. J Thromb Thrombolysis. 2010;30:220–5.
Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84:326–31.
Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Eng J Med. 2009;360:753–64.
Johnson JA, Caudle KE, Gong L, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017;102:397–404.
Rathore SS, Agarwal SK, Pande S, Singh SK, Mittal T, Mittal B. Therapeutic dosing of acenocoumarol: proposal of a population specific pharmacogenetic dosing algorithm and its validation in north Indians. PLoS One. 2012;7:e37844.
French B, Wang L, Gage BF, Horenstein RB, Limdi NA, Kimmel SE. A systematic analysis and comparison of warfarin initiation strategies. Pharmacogenet Genomics. 2016;26:445–52.
Kimmel SE. Warfarin pharmacogenomics: current best evidence. J Thromb Haemost. 2015;13:S266–71.
Krishna Kumar D, Shewade DG, Loriot MA, et al. An acenocoumarol dosing algorithm exploiting clinical and genetic factors in South Indian (Dravidian) population. Eur J Clin Pharmacol. 2015;71:173–81.
Gaikwad T, Ghosh K, Avery P, Kamali F, Shetty S. Warfarin dose model for the prediction of stable maintenance dose in Indian patients. Clin Appl Thromb Hemost. 2018;24:353–9.
Rathore SS, Agarwal SK, Pande S, Singh SK, Mittal T, Mittal B. Pharmacogenetic aspects of coumarinic oral anticoagulant therapies. Indian J Clin Biochem. 2011;26:222–9.
Shalia KK, Doshi SM, Parikh S, et al. Prevalence of VKORC1 and CYP2C9 gene polymorphisms in Indian population and its effect on warfarin response. J Assoc Physicians India. 2012;60:34–8.
Pavani A, Naushad SM, Rupasree Y, et al. Optimization of warfarin dose by population specific pharmacogenomic algorithm. Pharmacogenomics J. 2012;12:306–11.
Nahar R, Deb R, Saxena R, Puri RD, Verma IC. Variability in CYP2C9 allele frequency: a pilot study of its predicted impact on warfarin response among healthy South and North Indians. Pharmacol Rep. 2013;65:187–94.
Weckx S, Del-Favero J, Rademakers R, et al. novoSNP, a novel computational tool for sequence variation discovery. Genome Res. 2005;15:436–42.
Van Schie RM, Wessels JA, le Cessie S, et al. Loading and maintenance dose algorithms for phenprocoumon and acenocoumarol using patient characteristics and pharmacogenetic data. Eur Heart J. 2011;32:1909–17.
Khoury G, Sheikh-Taha M. Effect of age and sex on warfarin dosing. Clin Pharmacol. 2014;6:103–6.
Shi C, Yan W, Wang G, Wang F, Li Q, Lin N. Pharmacogenetics-based versus conventional dosing of warfarin: a meta-analysis of randomized controlled trials. PloS One. 2015;10:e0144511.
Acknowledgments
We acknowledge contributions of Dr. Dhananjay Raje and Ms. Moumita Chakraborty from MDS Bioanalytics, Pune, for conducting the statistical analysis. Blood samples for genotyping were analyzed free of cost at Mendelian Health Technologies Pvt. Ltd., Pune, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mathew A B, Hote MP, Talwar S, Rajashekhar P, and Choudhary SK have no conflict of interest. One of the authors (Parhar A) is employed with a commercial clinical genomic services organization (Mendelian Health Technologies, Pune, India) and was involved in the design, analysis, and discussion of findings. This study was a collaborative project between All India Institute of Medical Sciences, New Delhi, India, and Mendelian Health Technologies, Pune, India. No financial relationship exists between any organizations that might have interest in the submitted work.
Ethical approval for research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from institutional ethics committee.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choudhary, S.K., Mathew, A.B., Parhar, A. et al. Genetic polymorphisms and dosing of vitamin K antagonist in Indian patients after heart valve surgery. Indian J Thorac Cardiovasc Surg 35, 539–547 (2019). https://doi.org/10.1007/s12055-019-00812-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-019-00812-3