Abstract
Purpose
We studied the role of two powerful molecular signalling mechanisms involved in the cardioprotective effect of sphingosine-1-phosphate (S1P), a major component of high density lipoprotein (HDL) against myocardial ischaemic-reperfusion injury, namely the RISK pathway (Akt/Erk), including its downstream target FOXO-1 and, the SAFE pathway (TNF/STAT-3).
Methods
Control hearts from wildtype, TNF deficient (TNF−/−) or cardiomyocyte STAT-3 deficient (STAT-3−/−) male mice were perfused on a Langendorff apparatus (35 min global ischaemia and 45 min reperfusion). S1P (10 nM) was given at the onset of reperfusion for the first 7 min, with/without STAT-3 or Akt inhibitors, AG490 and wortmannin (W), respectively.
Results
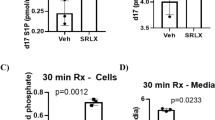
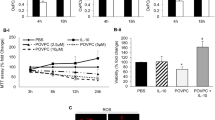
S1P reduced myocardial infarct size in wildtype hearts (39.3 ± 4.4% in control vs 17.3 ± 3.1% in S1P-treated hearts; n ≥ 6; p < 0.05) but not in STAT-3−/− or TNF−/− mice (34.2 ± 4.3% in STAT-3−/− and 34.1 ± 2.0% in TNF−/− mice; n ≥ 6; p = ns vs. their respective control). Both STAT-3 and Akt inhibitors abolished the protective effects of S1P (33.7 ± 3.3% in S1P + AG490 and 36.6 ± 4.9% in S1P + W; n = 6; p = ns vs. their respective control). Increased nuclear levels of phosphorylated STAT-3 (pSTAT-3), Akt and FOXO-1 were observed at 15 min reperfusion in wildtype mice with Western Blot analysis (53% STAT-3, 47% Akt, 41% FOXO-1; p < 0.05 vs control) but not in STAT-3−/− mice or in wiltype hearts treated with the Akt inhibitor. Interestingly, an activation of pSTAT-3 was noticed in the mitochondria at 7 min but not 15 min of reperfusion.
Conclusions
In conclusion, S1P activates both the SAFE and RISK pathways, therefore suggesting a dual protective signalling in S1P-induced cardioprotection.








Similar content being viewed by others
References
Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48:2325–33.
Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–18.
Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, et al. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–32.
Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–9.
Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of "modified reperfusion" protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–2.
Lecour S. Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40.
Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84:201–8.
Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croue A, Henrion D et al. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011
Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, et al. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase). Circulation. 2005;112:3911–8.
Smith RM, Suleman N, McCarthy J, Sack MN. Classic ischemic but not pharmacologic preconditioning is abrogated following genetic ablation of the TNFalpha gene. Cardiovasc Res. 2002;55:553–60.
Lacerda L, Somers S, Opie LH, Lecour S. Bradykinin, insulin and opioids mimic ischaemic postconditioning. Cardiovasc Res. 2010;87:S126.
Frias MA, James RW, Gerber-Wicht C, Lang U. Native and reconstituted HDL activate Stat3 in ventricular cardiomyocytes via ERK1/2: role of sphingosine-1-phosphate. Cardiovasc Res. 2009;82:313–23.
Smith RM, Suleman N, Lacerda L, Opie LH, Akira S, Chien KR, et al. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc Res. 2004;63:611–6.
Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352:809–15.
Berdyshev EV, Gorshkova IA, Garcia JG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal Biochem. 2005;339:129–36.
Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170–7.
Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–48.
Karliner JS. Mechanisms of cardioprotection by lysophospholipids. J Cell Biochem. 2004;92:1095–103.
Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314.
Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–9.
Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J Cardiovasc Pharmacol. 2009;53:189–97.
Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–51.
Kelly RF, Lamont KT, Somers S, Hacking D, Lacerda L, Thomas P, et al. Ethanolamine is a novel STAT-3 dependent cardioprotective agent. Basic Res Cardiol. 2010;105:763–70.
Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–85.
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7.
Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–85.
Lacerda L, McCarthy J, Mungly SF, Lynn EG, Sack MN, Opie LH, et al. TNFalpha protects cardiac mitochondria independently of its cell surface receptors. Basic Res Cardiol. 2010;105:751–62.
Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008;79:127–33.
Fuglesteg BN, Suleman N, Tiron C, Kanhema T, Lacerda L, Andreasen TV, et al. Signal transducer and activator of transcription 3 is involved in the cardioprotective signalling pathway activated by insulin therapy at reperfusion. Basic Res Cardiol. 2008;103:444–53.
Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–68.
Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–8.
Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–82.
Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–78.
Pedretti S, Raddatz E. STAT3alpha interacts with nuclear GSK3beta and cytoplasmic RISK pathway and stabilizes rhythm in the anoxic-reoxygenated embryonic heart. Basic Res Cardiol. 2011;106:355–69.
Negoro S, Kunisada K, Tone E, Funamoto M, Oh H, Kishimoto T, et al. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res. 2000;47:797–805.
Mascareno E, El-Shafei M, Maulik N, Sato M, Guo Y, Das DK, et al. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation. 2001;104:325–9.
Kurdi M, Booz GW. Can the protective actions of JAK-STAT in the heart be exploited therapeutically? Parsing the regulation of interleukin-6-type cytokine signaling. J Cardiovasc Pharmacol. 2007;50:126–41.
Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–8.
Acknowledgements
Part of this work was supported by the University of Cape Town, the National Research Foundation, the South African Medical Research Council and the Swiss-South African Research grant (PRJ #). Sarin J Somers was supported by Jan Minners Scholarship and Miguel Frias was supported by the Fonds National Suisse.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 125 kb)
Rights and permissions
About this article
Cite this article
Somers, S.J., Frias, M., Lacerda, L. et al. Interplay Between SAFE and RISK Pathways in Sphingosine-1-Phosphate–Induced Cardioprotection. Cardiovasc Drugs Ther 26, 227–237 (2012). https://doi.org/10.1007/s10557-012-6376-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-012-6376-2