Abstract
Purpose
Restenosis is a complex and heterogeneous pathophysiological phenomenon occurring in patients submitted to revascularization procedures. Previous studies proved the antirestenotic properties of injected allogenic mesenchymal stromal cells (MSCs) in an experimental model of rat carotid (re)stenosis induced through arteriotomy. In this study we describe some of the effects subsequent to MSC treatment of rats submitted to carotid arteriotomy and possibly responsible for their antirestenotic effect.
Methods
Rat MSCs were isolated from bone marrow, expanded in vitro and characterized. Subsequently, we evaluated the effects of MSC administration via tail vein at 3 and 7 days after carotid arteriotomy both in rat serum and in injured carotids, focusing on DNA oxidative damage (8-oxo-dG detection), cell proliferation index (BrdU incorporation assay), apoptotic index (TUNEL assay), the expression of inflammation- and proliferation-related genes (RT-PCR), the release of growth factors and of inflammation-related cytokines (antibody arrays and ELISA).
Results
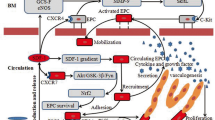
MSC administration induced a greater cell proliferation in carotids after arteriotomy, together with an increased level of VEGF in the serum and with the higher expression of VEGF mRNA in injured carotids. Serum analysis also revealed a decreased level of the pro-inflammatory cytokines CXCL1, CXCL5, L-Selectin, ICAM-1 and LIX, and of TIMP1 and SDF-1alpha in MSC-treated rats. The MSC immunomodulatory activity was confirmed by the decreased expression of TLR2 and TLR4 in injured carotids.
Conclusions
MSCs play an immunomodulatory paracrine role when injected in rats submitted to carotid arteriotomy, accompanied by the release of VEGF, possibly contributing to the accelerated repair of the injured vascular wall.





Similar content being viewed by others
References
Lal BK. Recurrent carotid stenosis after CEA and CAS: diagnosis and management. Semin Vasc Surg. 2007;20:259–66.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, et al. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–21.
Yokokawa M, Ohnishi S, Ishibashi-Ueda H, Obata H, Otani K, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves atrioventricular conduction in a rat model of complete atrioventricular block. Cell Transplant. 2008;17:1145–55.
Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, et al. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol. 2010;299:H1428–38.
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84.
Forte A, Di Micco G, Galderisi U, Guarino FM, Cipollaro M, De Feo M, et al. Molecular analysis of arterial stenosis in rat carotids. J Cell Physiol. 2001;186:307–13.
Forte A, Finicelli M, Mattia M, Berrino L, Rossi F, De Feo M, et al. Mesenchymal stem cells effectively reduce surgically induced stenosis in rat carotids. J Cell Physiol. 2008;217:789–99.
Forte A, Finicelli M, Grossi M, Vicchio M, Alessio N, Sante P, et al. DNA damage and repair in a model of rat vascular injury. Clin Sci (Lond). 2009. doi: 10.1042/SC20090416.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 2006;19:491–505.
Forte A, Finicelli M, De Luca P, Quarto C, Onorati F, Sante P, et al. Expression profiles in surgically-induced carotid stenosis: a combined transcriptomic and proteomic investigation. J Cell Mol Med. 2008;12:1956–73.
Li D, Zhang C, Song F, Lubenec I, Tian Y, Song QH. VEGF regulates FGF-2 and TGF-beta1 expression in injury endothelial cells and mediates smooth muscle cells proliferation and migration. Microvasc Res. 2009;77:134–42.
Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, Strijder CF, et al. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–8.
Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–24.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886.
Salvolini E, Orciani M, Vignini A, Mattioli-Belmonte M, Mazzanti L, Di Primio R. Skin-derived mesenchymal stem cells (S-MSCs) induce endothelial cell activation by paracrine mechanisms. Exp Dermatol. 19:848–50.
Novo E, Busletta C, Bonzo LV, Povero D, Paternostro C, Mareschi K, et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J Hepatol. 54:964–74.
Song H, Cha MJ, Song BW, Kim IK, Chang W, Lim S, et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 28:555–63.
Lanza C, Morando S, Voci A, Canesi L, Principato MC, Serpero LD, et al. Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J Neurochem. 2009;110:1674–84.
Witherick J, Wilkins A, Scolding N, Kemp K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2011:164608.
Tsubokawa T, Yagi K, Nakanishi C, Zuka M, Nohara A, Ino H, et al. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. 298:H1320-9.
Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–6.
Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–10.
Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, et al. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One. 6:e14486.
Sadat S, Gehmert S, Song YH, Yen Y, Bai X, Gaiser S, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363:674–9.
Hutter R, Carrick FE, Valdiviezo C, Wolinsky C, Rudge JS, Wiegand SJ, et al. Vascular endothelial growth factor regulates reendothelialization and neointima formation in a mouse model of arterial injury. Circulation. 2004;110:2430–5.
Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, et al. Local delivery of vascular endothelial growth factor accelerates reendothelialization and attenuates intimal hyperplasia in balloon-injured rat carotid artery. Circulation. 1995;91:2793–801.
Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, et al. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008;295:H2308–14.
Cobellis G, Maione C, Botti C, Coppola A, Silvestroni A, Lillo S, et al. Beneficial effects of VEGF secreted from stromal cells in supporting endothelial cell functions: therapeutic implications for critical limb ischemia. Cell Transplant. 2010;19:1425–37.
Bakondi B, Shimada IS, Peterson BM, Spees JL. SDF-1alpha secreted by human CD133-derived multipotent stromal cells promotes neural progenitor cell survival through CXCR7. Stem Cells Dev. 2011;20:1021–9.
Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52.
Schinkothe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206.
Yin Y, Zhao X, Fang Y, Yu S, Zhao J, Song M, et al. SDF-1alpha involved in mobilization and recruitment of endothelial progenitor cells after arterial injury in mice. Cardiovasc Pathol. 2010;19:218–27.
Li JM, Zhang X, Nelson PR, Odgren PR, Nelson JD, Vasiliu C, et al. Temporal evolution of gene expression in rat carotid artery following balloon angioplasty. J Cell Biochem. 2007;101:399–410.
Deng ZR, Yang C, Ma AQ, Chen XY, Geng T. Dynamic changes of plasma VEGF, SDF-1 and peripheral CD34+ cells in patients with acute myocardial infarction. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:1637–40.
Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72.
Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113.
Taube ME, Liu XW, Fridman R, Kim HR. TIMP-1 regulation of cell cycle in human breast epithelial cells via stabilization of p27(KIP1) protein. Oncogene. 2006;25:3041–8.
Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, et al. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–14.
Choong ML, Yong YP, Tan AC, Luo B, Lodish HF. LIX: a chemokine with a role in hematopoietic stem cells maintenance. Cytokine. 2004;25:239–45.
Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;
Halwani R, Al-Abri J, Beland M, Al-Jahdali H, Halayko AJ, Lee TH, et al. CC and CXC chemokines induce airway smooth muscle proliferation and survival. J Immunol. 2011;186:4156–63.
Acknowledgements
We are grateful to Dr. Karen English for support with ELISA serum analysis, to Dr. Monica Mattia for excellent care of animal welfare and to Ms. M.R. Cipollaro for administrative assistance.
Grants
This work has been partially funded by a SHRO grant to U.G.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Representative cross-sections of carotids from DiI-labeled MSC-treated rats harvested at 3 and 7 days after arteriotomy. A, Uninjured carotid; B, rat carotid harvested at 7 days after arteriotomy and DiI-labeled MSCs-treatement. Representative DiI-labeled MSCs emitting red fluorescence are indicated by white arrows. Nuclei were counterstained with Hoechst 33258, emitting blue fluorescence. 40x magnification. (JPEG 11 kb)
Supplemental Table 1
Summary of RT-PCR primer sequences, position, annealing temperature and PCR product length of the target genes analysed in rat carotids. (DOC 38 kb)
Supplemental Table 2
Summary of data concerning the characterization of bone marrow-derived MSCs expanded in vitro. TOP: RT-PCR analysis of PPAR-g and osteopontin on mRNA samples extracted from undifferentiated MSCs and from MSCs induced to differentiate to adipocytes and osteocytes with different media. Data are expressed as arbitrary densitometric units, mean ± SEM, n = 3 for each group. BOTTOM: FACS analysis of MSCs cultured for 15 days from passage zero. (DOC 36 kb)
Rights and permissions
About this article
Cite this article
Forte, A., Rinaldi, B., Sodano, L. et al. Stem Cell Therapy for Arterial Restenosis: Potential Parameters Contributing to the Success of Bone Marrow-Derived Mesenchymal Stromal Cells. Cardiovasc Drugs Ther 26, 9–21 (2012). https://doi.org/10.1007/s10557-011-6359-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-011-6359-8