Abstract
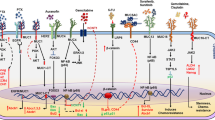
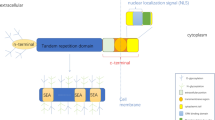
There has been a surge of interest in recent years in understanding the intricate mechanisms underlying cancer progression and treatment resistance. One molecule that has recently emerged in these mechanisms is MUC13 mucin, a transmembrane glycoprotein. Researchers have begun to unravel the molecular complexity of MUC13 and its impact on cancer biology. Studies have shown that MUC13 overexpression can disrupt normal cellular polarity, leading to the acquisition of malignant traits. Furthermore, MUC13 has been associated with increased cancer plasticity, allowing cells to undergo epithelial-mesenchymal transition (EMT) and metastasize. Notably, MUC13 has also been implicated in the development of chemoresistance, rendering cancer cells less responsive to traditional treatment options. Understanding the precise role of MUC13 in cellular plasticity, and chemoresistance could pave the way for the development of targeted therapies to combat cancer progression and enhance treatment efficacy.




Similar content being viewed by others
References
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249. https://doi.org/10.3322/caac.21660
Urruticoechea, A., Alemany, R., Balart, J., Villanueva, A., Viñals, F., & Capellá, G. (2010). Recent advances in cancer therapy: An overview. Current Pharmaceutical Design, 16, 3–10. https://doi.org/10.2174/138161210789941847
Baskar, R., Lee, K. A., Yeo, R., & Yeoh, K. W. (2012). Cancer and radiation therapy: Current advances and future directions. International Journal of Medical Sciences, 9, 193–199. https://doi.org/10.7150/ijms.3635
Wang, X., Zhang, H., & Chen, X. (2019). Drug resistance and combating drug resistance in cancer. Cancer Drug Resist, 2, 141–160. https://doi.org/10.20517/cdr.2019.10
Longley, D. B., & Johnston, P. G. (2005). Molecular mechanisms of drug resistance. The Journal of Pathology, 205, 275–292. https://doi.org/10.1002/path.1706
Smith, L., Watson, M. B., O’Kane, S. L., Drew, P. J., Lind, M. J., & Cawkwell, L. (2006). The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Molecular Cancer Therapeutics, 5, 2115–2120. https://doi.org/10.1158/1535-7163.Mct-06-0190
Harris, L. N., Broadwater, G., Lin, N. U., Miron, A., Schnitt, S. J., Cowan, D., Lara, J., Bleiweiss, I., Berry, D., Ellis, M., et al. (2006). Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: Results from CALGB 9342. Breast Cancer Research, 8, R66. https://doi.org/10.1186/bcr1622
Murray, S., Briasoulis, E., Linardou, H., Bafaloukos, D., & Papadimitriou, C. (2012). Taxane resistance in breast cancer: Mechanisms, predictive biomarkers and circumvention strategies. Cancer Treatment Reviews, 38, 890–903. https://doi.org/10.1016/j.ctrv.2012.02.011
Szakács, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C., & Gottesman, M. M. (2006). Targeting multidrug resistance in cancer. Nature Reviews Drug Discovery, 5, 219–234. https://doi.org/10.1038/nrd1984
Vulsteke, C., Pfeil, A. M., Schwenkglenks, M., Pettengell, R., Szucs, T. D., Lambrechts, D., Peeters, M., van Dam, P., Dieudonné, A. S., Hatse, S., et al. (2014). Impact of genetic variability and treatment-related factors on outcome in early breast cancer patients receiving (neo-) adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide, and docetaxel. Breast Cancer Research and Treatment, 147, 557–570. https://doi.org/10.1007/s10549-014-3105-5
Porkka, K., Blomqvist, C., Rissanen, P., Elomaa, I., & Pyrhönen, S. (1994). Salvage therapies in women who fail to respond to first-line treatment with fluorouracil, epirubicin, and cyclophosphamide for advanced breast cancer. Journal of Clinical Oncology, 12, 1639–1647. https://doi.org/10.1200/jco.1994.12.8.1639
Sládek, N. E., Kollander, R., Sreerama, L., & Kiang, D. T. (2002). Cellular levels of aldehyde dehydrogenases (ALDH1A1 and ALDH3A1) as predictors of therapeutic responses to cyclophosphamide-based chemotherapy of breast cancer: A retrospective study. Rational individualization of oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer Chemotherapy and Pharmacology, 49, 309–321. https://doi.org/10.1007/s00280-001-0412-4
Galluzzi, L., Vitale, I., Michels, J., Brenner, C., Szabadkai, G., Harel-Bellan, A., Castedo, M., & Kroemer, G. (2014). Systems biology of cisplatin resistance: past, present and future. Cell Death Dis, 5, e1257. https://doi.org/10.1038/cddis.2013.428
Mansoori, B., Mohammadi, A., Davudian, S., Shirjang, S., & Baradaran, B. (2017). The different mechanisms of cancer drug resistance: A brief review. Advanced Pharmaceutical Bulletin, 7, 339–348. https://doi.org/10.15171/apb.2017.041
Chang, J. C. (2016). Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine (Baltimore), 95, S20-s25. https://doi.org/10.1097/md.0000000000004766
Chen, K., Huang, Y. H., & Chen, J. L. (2013). Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharmacologica Sinica, 34, 732–740. https://doi.org/10.1038/aps.2013.27
Prasad, S., Ramachandran, S., Gupta, N., Kaushik, I., & Srivastava, S. K. (2020). Cancer cells stemness: A doorstep to targeted therapy. Biochimica et Biophysica Acta, Molecular Basis of Disease, 1866, 165424. https://doi.org/10.1016/j.bbadis.2019.02.019
Furth, J., Kahn, M. C., & Breedis, C. (1937). The transmission of leukemia of mice with a single cell. The American Journal of Cancer, 31, 276–282.
Dick, J. E. (2008). Stem cell concepts renew cancer research. Blood, The Journal of the American Society of Hematology, 112, 4793–4807.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nature Reviews Cancer, 8, 755–768.
Iwata, M. (1968). Study of serum lipids in infants. 1. Serum lipids in healthy infants. Nihon Shonika Gakkai Zasshi Acta Paediatrica Japonica, 72, 1075–1081.
Phi, L.T.H., Sari, I.N., Yang, Y.-G., Lee, S.-H., Jun, N., Kim, K.S., Lee, Y.K., Kwon, H.Y. (2018). Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells International, 2018, 5416923. https://doi.org/10.1155/2018/5416923
Shervington, A., & Lu, C. (2008). Expression of multidrug resistance genes in normal and cancer stem cells. Cancer Investigation, 26, 535–542.
Vinogradov, S., & Wei, X. (2012). Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine, 7, 597–615.
Rachagani, S., Torres, M. P., Moniaux, N., & Batra, S. K. (2009). Current status of mucins in the diagnosis and therapy of cancer. BioFactors, 35, 509–527. https://doi.org/10.1002/biof.64
Reynolds, I. S., Fichtner, M., McNamara, D. A., Kay, E. W., Prehn, J. H. M., & Burke, J. P. (2019). Mucin glycoproteins block apoptosis; promote invasion, proliferation, and migration; and cause chemoresistance through diverse pathways in epithelial cancers. Cancer and Metastasis Reviews, 38, 237–257. https://doi.org/10.1007/s10555-019-09781-w
Dhanisha, S. S., Guruvayoorappan, C., Drishya, S., & Abeesh, P. (2018). Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Critical Reviews in Oncology Hematology, 122, 98–122. https://doi.org/10.1016/j.critrevonc.2017.12.006
Brockhausen, I. (2003). Glycodynamics of mucin biosynthesis in gastrointestinal tumor cells. Advances in Experimental Medicine and Biology, 535, 163–188. https://doi.org/10.1007/978-1-4615-0065-0_11
Yonezawa, S., Higashi, M., Yamada, N., Yokoyama, S., Kitamoto, S., Kitajima, S., & Goto, M. (2011). Mucins in human neoplasms: Clinical pathology, gene expression and diagnostic application. Pathology International, 61, 697–716. https://doi.org/10.1111/j.1440-1827.2011.02734.x
Nath, S., & Mukherjee, P. (2014). MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends in Molecular Medicine, 20, 332–342. https://doi.org/10.1016/j.molmed.2014.02.007
Moniaux, N., Escande, F., Porchet, N., Aubert, J. P., & Batra, S. K. (2001). Structural organization and classification of the human mucin genes. Frontiers in Bioscience, 6, D1192-1206. https://doi.org/10.2741/moniaux
van Putten, J. P. M., & Strijbis, K. (2017). Transmembrane mucins: Signaling receptors at the intersection of inflammation and cancer. Journal of Innate Immunity, 9, 281–299. https://doi.org/10.1159/000453594
Chauhan, S. C., Singh, A. P., Ruiz, F., Johansson, S. L., Jain, M., Smith, L. M., Moniaux, N., & Batra, S. K. (2006). Aberrant expression of MUC4 in ovarian carcinoma: Diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Modern Pathology, 19, 1386–1394. https://doi.org/10.1038/modpathol.3800646
Hollingsworth, M. A., & Swanson, B. J. (2004). Mucins in cancer: Protection and control of the cell surface. Nature Reviews Cancer, 4, 45–60. https://doi.org/10.1038/nrc1251
Andrianifahanana, M., Moniaux, N., Schmied, B. M., Ringel, J., Friess, H., Hollingsworth, M. A., Büchler, M. W., Aubert, J. P., & Batra, S. K. (2001). Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: A potential role of MUC4 as a tumor marker of diagnostic significance. Clinical Cancer Research, 7, 4033–4040.
Balagué, C., Audié, J. P., Porchet, N., & Real, F. X. (1995). In situ hybridization shows distinct patterns of mucin gene expression in normal, benign, and malignant pancreas tissues. Gastroenterology, 109, 953–964. https://doi.org/10.1016/0016-5085(95)90406-9
Dong, Y., Walsh, M. D., Cummings, M. C., Wright, R. G., Khoo, S. K., Parsons, P. G., & McGuckin, M. A. (1997). Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. The Journal of Pathology, 183, 311–317. https://doi.org/10.1002/(sici)1096-9896(199711)183:3%3c311::Aid-path917%3e3.0.Co;2-2
Giuntoli, R. L., Rodriguez, G. C., Whitaker, R. S., Dodge, R., & Voynow, J. A. (1998). Mucin gene expression in ovarian cancers. Cancer Research, 58, 5546–5550.
Maher, D. M., Gupta, B. K., Nagata, S., Jaggi, M., & Chauhan, S. C. (2011). Mucin 13: Structure, function, and potential roles in cancer pathogenesis. Molecular Cancer Research, 9, 531–537. https://doi.org/10.1158/1541-7786.Mcr-10-0443
Jonckheere, N., Skrypek, N., & Van Seuningen, I. (2014). Mucins and tumor resistance to chemotherapeutic drugs. Biochimica et Biophysica Acta, 1846, 142–151. https://doi.org/10.1016/j.bbcan.2014.04.008
Lee, D.H., Choi, S., Park, Y., Jin, H.S. (2021). Mucin1 and Mucin16: Therapeutic targets for cancer therapy. Pharmaceuticals (Basel), 14. https://doi.org/10.3390/ph14101053.
Ponnusamy, M. P., Seshacharyulu, P., Lakshmanan, I., Vaz, A. P., Chugh, S., & Batra, S. K. (2013). Emerging role of mucins in epithelial to mesenchymal transition. Current Cancer Drug Targets, 13, 945–956. https://doi.org/10.2174/15680096113136660100
Marimuthu, S., Rauth, S., Ganguly, K., Zhang, C., Lakshmanan, I., Batra, S. K., & Ponnusamy, M. P. (2021). Mucins reprogram stemness, metabolism and promote chemoresistance during cancer progression. Cancer and Metastasis Reviews, 40, 575–588. https://doi.org/10.1007/s10555-021-09959-1
Gendler, S. J., & Spicer, A. P. (1995). Epithelial mucin genes. Annual Review of Physiology, 57, 607–634. https://doi.org/10.1146/annurev.ph.57.030195.003135
Thornton, D. J., Rousseau, K., & McGuckin, M. A. (2008). Structure and function of the polymeric mucins in airways mucus. Annual Review of Physiology, 70, 459–486. https://doi.org/10.1146/annurev.physiol.70.113006.100702
Desseyn, J.-L., Gouyer, V., Tetaert, T. (2008). Architecture of the gel-forming mucins. The Epithelial Mucins: Structure/Function. Roles in Cancer and Inflammatory Diseases, Isabelle Van Seuningen/Research Signpost, 1–16, 978-81-308-0256-5. ⟨hal-02340996⟩.
Hattrup, C. L., & Gendler, S. J. (2008). Structure and function of the cell surface (tethered) mucins. Annual Review of Physiology, 70, 431–457. https://doi.org/10.1146/annurev.physiol.70.113006.100659
Jonckheere, N., Skrypek, N., Frénois, F., & Van Seuningen, I. (2013). Membrane-bound mucin modular domains: From structure to function. Biochimie, 95, 1077–1086. https://doi.org/10.1016/j.biochi.2012.11.005
Jonckheere, N., & Van Seuningen, I. (2008). The membrane-bound mucins: How large O-glycoproteins play key roles in epithelial cancers and hold promise as biological tools for gene-based and immunotherapies. Critical Reviews in Oncogenesis, 14, 177–196. https://doi.org/10.1615/critrevoncog.v14.i2-3.30
Segui-Perez, C., Stapels, D.A.C., Ma, Z., Su, J., Passchier, E., Westendorp, B., Wu, W., Putten, J.P.M.V., Strijbis, K. (2022). MUC13 negatively regulates tight junction proteins and intestinal epithelial barrier integrity via Protein Kinase C. bioRxiv,. https://doi.org/10.1101/2022.10.27.51398
Williams, S. J., Wreschner, D. H., Tran, M., Eyre, H. J., Sutherland, G. R., & McGuckin, M. A. (2001). Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. Journal of Biological Chemistry, 276, 18327–18336. https://doi.org/10.1074/jbc.M008850200
Chauhan, S. C., Ebeling, M. C., Maher, D. M., Koch, M. D., Watanabe, A., Aburatani, H., Lio, Y., & Jaggi, M. (2012). MUC13 mucin augments pancreatic tumorigenesis. Molecular Cancer Therapeutics, 11, 24–33. https://doi.org/10.1158/1535-7163.Mct-11-0598
Chauhan, S. C., Kumar, D., & Jaggi, M. (2009). Mucins in ovarian cancer diagnosis and therapy. Journal of Ovarian Research, 2, 21. https://doi.org/10.1186/1757-2215-2-21
Chauhan, S. C., Vannatta, K., Ebeling, M. C., Vinayek, N., Watanabe, A., Pandey, K. K., Bell, M. C., Koch, M. D., Aburatani, H., Lio, Y., et al. (2009). Expression and functions of transmembrane mucin MUC13 in ovarian cancer. Cancer Research, 69, 765–774. https://doi.org/10.1158/0008-5472.Can-08-0587
Gupta, B. K., Maher, D. M., Ebeling, M. C., Stephenson, P. D., Puumala, S. E., Koch, M. R., Aburatani, H., Jaggi, M., & Chauhan, S. C. (2014). Functions and regulation of MUC13 mucin in colon cancer cells. Journal of Gastroenterology, 49, 1378–1391. https://doi.org/10.1007/s00535-013-0885-z
Khan, S., Ebeling, M. C., Zaman, M. S., Sikander, M., Yallapu, M. M., Chauhan, N., Yacoubian, A. M., Behrman, S. W., Zafar, N., Kumar, D., et al. (2014). MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget, 5, 7599–7609. https://doi.org/10.18632/oncotarget.2281
Khan, S., Sikander, M., Ebeling, M. C., Ganju, A., Kumari, S., Yallapu, M. M., Hafeez, B. B., Ise, T., Nagata, S., Zafar, N., et al. (2017). MUC13 interaction with receptor tyrosine kinase HER2 drives pancreatic ductal adenocarcinoma progression. Oncogene, 36, 491–500. https://doi.org/10.1038/onc.2016.218
Filippou, P. S., Ren, A. H., Korbakis, D., Dimitrakopoulos, L., Soosaipillai, A., Barak, V., Frenkel, S., Pe’er, J., Lotem, M., Merims, S., et al. (2018). Exploring the potential of mucin 13 (MUC13) as a biomarker for carcinomas and other diseases. Clinical Chemistry and Laboratory Medicine, 56, 1945–1953. https://doi.org/10.1515/cclm-2018-0139
Khan, S., Zafar, N., Khan, S. S., Setua, S., Behrman, S. W., Stiles, Z. E., Yallapu, M. M., Sahay, P., Ghimire, H., Ise, T., et al. (2018). Clinical significance of MUC13 in pancreatic ductal adenocarcinoma. HPB: The Official Journal of the International Hepato Pancreato Biliary Association, 20, 563–572. https://doi.org/10.1016/j.hpb.2017.12.003
Kumari, S., Khan, S., Gupta, S. C., Kashyap, V. K., Yallapu, M. M., Chauhan, S. C., & Jaggi, M. (2018). MUC13 contributes to rewiring of glucose metabolism in pancreatic cancer. Oncogenesis, 7, 19. https://doi.org/10.1038/s41389-018-0031-0
Massey, A. E., Doxtater, K. A., Yallapu, M. M., & Chauhan, S. C. (2020). Biophysical changes caused by altered MUC13 expression in pancreatic cancer cells. Micron, 130, 102822. https://doi.org/10.1016/j.micron.2019.102822
Nishii, Y., Yamaguchi, M., Kimura, Y., Hasegawa, T., Aburatani, H., Uchida, H., Hirata, K., & Sakuma, Y. (2015). A newly developed anti-Mucin 13 monoclonal antibody targets pancreatic ductal adenocarcinoma cells. International Journal of Oncology, 46, 1781–1787. https://doi.org/10.3892/ijo.2015.2880
Stiles, Z. E., Khan, S., Patton, K. T., Jaggi, M., Behrman, S. W., & Chauhan, S. C. (2019). Transmembrane mucin MUC13 distinguishes intraductal papillary mucinous neoplasms from non-mucinous cysts and is associated with high-risk lesions. HPB: The Official Journal of the International Hepato Pancreato Biliary Association, 21, 87–95. https://doi.org/10.1016/j.hpb.2018.07.009
Mito, K., Saito, M., Morita, K., Maetani, I., Sata, N., Mieno, M., & Fukushima, N. (2018). Clinicopathological and prognostic significance of MUC13 and AGR2 expression in intraductal papillary mucinous neoplasms of the pancreas. Pancreatology, 18, 407–412. https://doi.org/10.1016/j.pan.2018.04.003
Gupta, B. K., Maher, D. M., Ebeling, M. C., Sundram, V., Koch, M. D., Lynch, D. W., Bohlmeyer, T., Watanabe, A., Aburatani, H., Puumala, S. E., et al. (2012). Increased expression and aberrant localization of mucin 13 in metastatic colon cancer. Journal of Histochemistry and Cytochemistry, 60, 822–831. https://doi.org/10.1369/0022155412460678
Walsh, M. D., Young, J. P., Leggett, B. A., Williams, S. H., Jass, J. R., & McGuckin, M. A. (2007). The MUC13 cell surface mucin is highly expressed by human colorectal carcinomas. Human Pathology, 38, 883–892. https://doi.org/10.1016/j.humpath.2006.11.020
Kasprzak, A., Adamek, A. (2019). Mucins: The Old, the new and the promising factors in hepatobiliary carcinogenesis. International Journal of Molecular Sciences, 20. https://doi.org/10.3390/ijms20061288
Tiemin, P., Fanzheng, M., Peng, X., Jihua, H., Ruipeng, S., Yaliang, L., Yan, W., Junlin, X., Qingfu, L., Zhefeng, H., et al. (2020). MUC13 promotes intrahepatic cholangiocarcinoma progression via EGFR/PI3K/AKT pathways. Journal of Hepatology, 72, 761–773. https://doi.org/10.1016/j.jhep.2019.11.021
Dai, Y., Liu, L., Zeng, T., Liang, J. Z., Song, Y., Chen, K., Li, Y., Chen, L., Zhu, Y. H., Li, J., et al. (2018). Overexpression of MUC13, a poor prognostic predictor, promotes cell growth by activating wnt signaling in hepatocellular carcinoma. American Journal of Pathology, 188, 378–391. https://doi.org/10.1016/j.ajpath.2017.10.016
Pang, Y., Zhang, Y., Zhang, H. Y., Wang, W. H., Jin, G., Liu, J. W., & Zhu, Z. J. (2022). MUC13 promotes lung cancer development and progression by activating ERK signaling. Oncology Letters, 23, 37. https://doi.org/10.3892/ol.2021.13155
Sheng, Y., Ng, C. P., Lourie, R., Shah, E. T., He, Y., Wong, K. Y., Seim, I., Oancea, I., Morais, C., Jeffery, P. L., et al. (2017). MUC13 overexpression in renal cell carcinoma plays a central role in tumor progression and drug resistance. International Journal of Cancer, 140, 2351–2363. https://doi.org/10.1002/ijc.30651
Xu, Z., Liu, Y., Yang, Y., Wang, J., Zhang, G., Liu, Z., Fu, H., Wang, Z., Liu, H., & Xu, J. (2017). High expression of Mucin13 associates with grimmer postoperative prognosis of patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget, 8, 7548–7558. https://doi.org/10.18632/oncotarget.13692
Shimamura, T., Ito, H., Shibahara, J., Watanabe, A., Hippo, Y., Taniguchi, H., Chen, Y., Kashima, T., Ohtomo, T., Tanioka, F., et al. (2005). Overexpression of MUC13 is associated with intestinal-type gastric cancer. Cancer Science, 96, 265–273. https://doi.org/10.1111/j.1349-7006.2005.00043.x
He, L., Qu, L., Wei, L., Chen, Y., & Suo, J. (2017). Reduction of miR-132-3p contributes to gastric cancer proliferation by targeting MUC13. Molecular Medicine Reports, 15, 3055–3061. https://doi.org/10.3892/mmr.2017.6347
Zhao, Z. T., Li, Y., Yuan, H. Y., Ma, F. H., Song, Y. M., & Tian, Y. T. (2020). Identification of key genes and pathways in gastric signet ring cell carcinoma based on transcriptome analysis. World J Clin Cases, 8, 658–669. https://doi.org/10.12998/wjcc.v8.i4.658
Wang, H., Shen, L., Lin, Y., Shi, Q., Yang, Y., & Chen, K. (2015). The expression and prognostic significance of Mucin 13 and Mucin 20 in esophageal squamous cell carcinoma. Journal of Cancer Research and Therapeutics, 11(Suppl 1), C74-79. https://doi.org/10.4103/0973-1482.163846
Li, P., Wang, H., Hou, M., Li, D., & Bai, H. (2017). Upstream stimulating factor1 (USF1) enhances the proliferation of glioblastoma stem cells mainly by activating the transcription of mucin13 (MUC13). Die Pharmazie, 72, 98–102. https://doi.org/10.1691/ph.2017.6788
Sung, H. Y., Park, A. K., Ju, W., & Ahn, J. H. (2014). Overexpression of mucin 13 due to promoter methylation promotes aggressive behavior in ovarian cancer cells. Yonsei Medical Journal, 55, 1206–1213. https://doi.org/10.3349/ymj.2014.55.5.1206
Dhasmana, A., Dhasmana, S., Agarwal, S., Khan, S., Haque, S., Jaggi, M., Yallapu, M. M., & Chauhan, S. C. (2023). Integrative big transcriptomics data analysis implicates crucial role of MUC13 in pancreatic cancer. Computational and Structural Biotechnology Journal, 21, 2845–2857. https://doi.org/10.1016/j.csbj.2023.04.029
Jonckheere, N., Vincent, A., Neve, B., & Van Seuningen, I. (2021). Mucin expression, epigenetic regulation and patient survival: A toolkit of prognostic biomarkers in epithelial cancers. Biochimica et Biophysica Acta - Reviews on Cancer, 1876, 188538. https://doi.org/10.1016/j.bbcan.2021.188538
Thompson, C. M., Cannon, A., West, S., Ghersi, D., Atri, P., Bhatia, R., Smith, L., Rachagani, S., Wichman, C., Kumar, S., et al. (2021). Mucin expression and splicing determine novel subtypes and patient mortality in pancreatic ductal adenocarcinoma. Clinical Cancer Research, 27, 6787–6799. https://doi.org/10.1158/1078-0432.Ccr-21-1591
Fabregas, J. C., Ramnaraign, B., & George, T. J. (2022). Clinical updates for colon cancer care in 2022. Clinical Colorectal Cancer, 21, 198–203. https://doi.org/10.1016/j.clcc.2022.05.006
Byrd, J. C., & Bresalier, R. S. (2004). Mucins and mucin binding proteins in colorectal cancer. Cancer and Metastasis Reviews, 23, 77–99. https://doi.org/10.1023/a:1025815113599
Jonckheere, N., & Van Seuningen, I. (2010). The membrane-bound mucins: From cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie, 92, 1–11. https://doi.org/10.1016/j.biochi.2009.09.018
Cox, K.E., Liu, S., Lwin, T.M., Hoffman, R.M., Batra, S.K., Bouvet, M. (2023). The mucin family of proteins: Candidates as potential biomarkers for colon cancer. Cancers (Basel), 15. https://doi.org/10.3390/cancers15051491.
Sheng, Y. H., He, Y., Hasnain, S. Z., Wang, R., Tong, H., Clarke, D. T., Lourie, R., Oancea, I., Wong, K. Y., Lumley, J. W., et al. (2017). MUC13 protects colorectal cancer cells from death by activating the NF-κB pathway and is a potential therapeutic target. Oncogene, 36, 700–713. https://doi.org/10.1038/onc.2016.241
Sheng, Y. H., Wong, K. Y., Seim, I., Wang, R., He, Y., Wu, A., Patrick, M., Lourie, R., Schreiber, V., Giri, R., et al. (2019). MUC13 promotes the development of colitis-associated colorectal tumors via β-catenin activity. Oncogene, 38, 7294–7310. https://doi.org/10.1038/s41388-019-0951-y
Doxtater, K., Tripathi, M. K., Sekhri, R., Hafeez, B. B., Khan, S., Zafar, N., Behrman, S. W., Yallapu, M. M., Jaggi, M., & Chauhan, S. C. (2023). MUC13 drives cancer aggressiveness and metastasis through the YAP1-dependent pathway. Life Sci Alliance, 6(12). https://doi.org/10.26508/lsa.202301975
Lei, Z. N., Teng, Q. X., Tian, Q., Chen, W., Xie, Y., Wu, K., Zeng, Q., Zeng, L., Pan, Y., Chen, Z. S., et al. (2022). Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduction and Targeted Therapy, 7, 358. https://doi.org/10.1038/s41392-022-01190-w
Stomach Cancer Survival Rates. The American Cancer Society medical and editorial content team 2023. https://www.cancer.org/cancer/types/stomach-cancer/detection-diagnosis-staging/survival-rates.html
Hepatocellular Carcinoma (HCC). Cleveland Clinic medical professional 2021. https://my.clevelandclinic.org/health/diseases/21709-hepatocellular-carcinoma-hcc
Toh, M. R., Wong, E. Y. T., Wong, S. H., Ng, A. W. T., Loo, L. H., Chow, P. K., & Ngeow, J. (2023). Global Epidemiology and genetics of hepatocellular carcinoma. Gastroenterology, 164, 766–782. https://doi.org/10.1053/j.gastro.2023.01.033
Ganesan, P., & Kulik, L. M. (2023). Hepatocellular carcinoma: New developments. Clinics in Liver Disease, 27, 85–102. https://doi.org/10.1016/j.cld.2022.08.004
Webb, P. M., & Jordan, S. J. (2017). Epidemiology of epithelial ovarian cancer. Best Practice & Research Clinical Obstetrics & Gynaecology, 41, 3–14. https://doi.org/10.1016/j.bpobgyn.2016.08.006
Ren, A. H., Filippou, P. S., Soosaipillai, A., Dimitrakopoulos, L., Korbakis, D., Leung, F., Kulasingam, V., Bernardini, M. Q., & Diamandis, E. P. (2023). Mucin 13 (MUC13) as a candidate biomarker for ovarian cancer detection: Potential to complement CA125 in detecting non-serous subtypes. Clinical Chemistry and Laboratory Medicine, 61, 464–472. https://doi.org/10.1515/cclm-2022-0491
Bade, B. C., & Dela Cruz, C. S. (2020). Lung cancer 2020: Epidemiology, etiology, and prevention. Clinics in Chest Medicine, 41, 1–24. https://doi.org/10.1016/j.ccm.2019.10.001
Yang, D., Liu, Y., Bai, C., Wang, X., & Powell, C. A. (2020). Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Letters, 468, 82–87. https://doi.org/10.1016/j.canlet.2019.10.009
Vaidya, F. U., Sufiyan Chhipa, A., Mishra, V., Gupta, V. K., Rawat, S. G., Kumar, A., & Pathak, C. (2022). Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep (Hoboken), 5, e1291. https://doi.org/10.1002/cnr2.1291
Barrera-Rodríguez, R. (2018). Importance of the Keap1-Nrf2 pathway in NSCLC: Is it a possible biomarker? Biomedical Reports, 9, 375–382. https://doi.org/10.3892/br.2018.1143
Hammerman, P. S., Lawrence, M. S., Voet, D., Jing, R., Cibulskis, K., Sivachenko, A., Stojanov, P., McKenna, A., Lander, E. S., Gabriel, S., Getz, G., Sougnez, C., Imielinski, M., Helman, E., Hernandez, B., Pho, N. H., Meyerson, M., Chu, A., Chun, H.-J. E., et al. Comprehensive genomic characterization of squamous cell lung cancers. PubMed. nih.gov
Giordano, T. J. (2014). The cancer genome atlas research network: A sight to behold. Endocrine Pathology, 25, 362–365. https://doi.org/10.1007/s12022-014-9345-4
Collisson, E. A., Campbell, J. D., Brooks, A. N., Berger, A. H., Lee, W., Chmielecki, J., Beer, D. G., Cope, L., Creighton, C. J., Danilova, L., Ding, L., Getz, G., Hammerman, P. S., Neil Hayes, D., Hernandez, B., Herman, J. G., Heymach, J. V., Jurisica, I., Kucherlapati, R., … John Flynn, H. (2014). Comprehensive molecular profiling of lung adenocarcinoma. Nature, 511(7511), 543–550. https://doi.org/10.1038/nature13385
Begicevic, R.R., Falasca, M. (2017). ABC transporters in cancer stem cells: Beyond chemoresistance. International Journal of Molecular Sciences, 18. https://doi.org/10.3390/ijms18112362.
Zhao, J. (2016). Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacology & Therapeutics, 160, 145–158. https://doi.org/10.1016/j.pharmthera.2016.02.008
Hasan, S., Taha, R., & Omri, H. E. (2018). Current opinions on chemoresistance: An overview. Bioinformation, 14, 80–85. https://doi.org/10.6026/97320630014080
Chen, W., Qin, Y., & Liu, S. (2018). Cytokines, breast cancer stem cells (BCSCs) and chemoresistance. Clinical and Translational Medicine, 7, 27. https://doi.org/10.1186/s40169-018-0205-6
Samuel, S.M., Varghese, E., Koklesová, L., Líšková, A., Kubatka, P., Büsselberg, D. (2020). Counteracting chemoresistance with metformin in breast cancers: Targeting cancer stem cells. Cancers (Basel), 12. https://doi.org/10.3390/cancers12092482.
Nussinov, R., Tsai, C. J., & Jang, H. (2017). A new view of pathway-driven drug resistance in tumor proliferation. Trends in Pharmacological Sciences, 38, 427–437. https://doi.org/10.1016/j.tips.2017.02.001
Rajabpour, A., Rajaei, F., & Teimoori-Toolabi, L. (2017). Molecular alterations contributing to pancreatic cancer chemoresistance. Pancreatology, 17, 310–320. https://doi.org/10.1016/j.pan.2016.12.013
Alfarouk, K. O., Stock, C. M., Taylor, S., Walsh, M., Muddathir, A. K., Verduzco, D., Bashir, A. H., Mohammed, O. Y., Elhassan, G. O., Harguindey, S., et al. (2015). Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell International, 15, 71. https://doi.org/10.1186/s12935-015-0221-1
Moulder, S. (2010). Intrinsic resistance to chemotherapy in breast cancer. Womens Health (Lond), 6, 821–830. https://doi.org/10.2217/whe.10.60
Lippert, T. H., Ruoff, H. J., & Volm, M. (2008). Intrinsic and acquired drug resistance in malignant tumors. The main reason for therapeutic failure. Arzneimittelforschung, 58, 261–264. https://doi.org/10.1055/s-0031-1296504
Schwarzenbach, H., & Gahan, P. B. (2019). Resistance to cis- and carboplatin initiated by epigenetic changes in ovarian cancer patients. Cancer Drug Resist, 2, 271–296. https://doi.org/10.20517/cdr.2019.010
Chen, Y., Song, Y., Mi, Y., Jin, H., Cao, J., Li, H., Han, L., Huang, T., Zhang, X., Ren, S., et al. (2020). microRNA-499a promotes the progression and chemoresistance of cervical cancer cells by targeting SOX6. Apoptosis, 25, 205–216. https://doi.org/10.1007/s10495-019-01588-y
Wang, H., Guan, Z., He, K., Qian, J., Cao, J., & Teng, L. (2017). LncRNA UCA1 in anti-cancer drug resistance. Oncotarget, 8, 64638–64650. https://doi.org/10.18632/oncotarget.18344
Ebrahimi Ghahnavieh, L., Tabatabaeian, H., Ebrahimi Ghahnavieh, Z., Honardoost, M. A., Azadeh, M., Moazeni Bistgani, M., & Ghaedi, K. (2020). Fluctuating expression of miR-584 in primary and high-grade gastric cancer. BMC Cancer, 20, 621. https://doi.org/10.1186/s12885-020-07116-5
Lim, S. K., Tabatabaeian, H., Lu, S. Y., Kang, S. A., Sundaram, G. M., Sampath, P., Chan, S. W., Hong, W. J., & Lim, Y. P. (2020). Hippo/MST blocks breast cancer by downregulating WBP2 oncogene expression via miRNA processor Dicer. Cell Death & Disease, 11, 669. https://doi.org/10.1038/s41419-020-02901-3
Adami, B., Tabatabaeian, H., Ghaedi, K., Talebi, A., Azadeh, M., & Dehdashtian, E. (2019). miR-146a is deregulated in gastric cancer. Journal of Cancer Research and Therapeutics, 15, 108–114. https://doi.org/10.4103/jcrt.JCRT_855_17
Chan, J. J., Tay, Y. (2018). Noncoding RNA:RNA regulatory networks in cancer. The International Journal of Molecular Science, 19. https://doi.org/10.3390/ijms19051310.
Anastasiadou, E., Jacob, L. S., & Slack, F. J. (2018). Non-coding RNA networks in cancer. Nature Reviews Cancer, 18, 5–18. https://doi.org/10.1038/nrc.2017.99
Ramos, E. K., Hoffmann, A. D., Gerson, S. L., & Liu, H. (2017). New opportunities and challenges to defeat cancer stem cells. Trends in cancer, 3, 780–796.
Singh, A., & Settleman, J. (2010). EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene, 29, 4741–4751.
Flanagan, D. J., Barker, N., Costanzo, N. S. D., Mason, E. A., Gurney, A., Meniel, V. S., Koushyar, S., Austin, C. R., Ernst, M., & Pearson, H. B. (2019). Frizzled-7 is required for Wnt signaling in gastric tumors with and without Apc mutations. Cancer Research, 79, 970–981.
Zhang, Y., Morris, J. P., IV., Yan, W., Schofield, H. K., Gurney, A., Simeone, D. M., Millar, S. E., Hoey, T., Hebrok, M., & Pasca di Magliano, M. (2013). Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Research, 73, 4909–4922.
Sinnberg, T., Levesque, M. P., Krochmann, J., Cheng, P. F., Ikenberg, K., Meraz-Torres, F., Niessner, H., Garbe, C., & Busch, C. (2018). Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Molecular Cancer, 17, 1–19.
Liu, Y., Chang, Y., Lu, S., & Xiang, Y. Y. (2019). Downregulation of long noncoding RNA DGCR5 contributes to the proliferation, migration, and invasion of cervical cancer by activating Wnt signaling pathway. Journal of Cellular Physiology, 234, 11662–11669.
Veeck, J., Bektas, N., Hartmann, A., Kristiansen, G., Heindrichs, U., Knüchel, R., & Dahl, E. (2008). Wnt signalling in human breast cancer: Expression of the putative Wnt inhibitor Dickkopf-3 (DKK3) is frequently suppressed by promoter hypermethylation in mammary tumours. Breast Cancer Research, 10, 1–11.
Murillo-Garzón, V., & Kypta, R. (2017). WNT signalling in prostate cancer. Nature Reviews Urology, 14, 683–696.
McCord, M., Mukouyama, Y.-S., Gilbert, M. R., & Jackson, S. (2017). Targeting WNT signaling for multifaceted glioblastoma therapy. Frontiers in cellular neuroscience, 11, 318.
van Andel, H., Kocemba, K. A., Spaargaren, M., & Pals, S. T. (2019). Aberrant Wnt signaling in multiple myeloma: Molecular mechanisms and targeting options. Leukemia, 33, 1063–1075.
Ashihara, E., Takada, T., & Maekawa, T. (2015). Targeting the canonical Wnt/β-catenin pathway in hematological malignancies. Cancer Science, 106, 665–671.
Yadav, A. K., & Desai, N. S. (2019). Cancer stem cells: Acquisition, characteristics, therapeutic implications, targeting strategies and future prospects. Stem Cell Reviews and Reports, 15, 331–355.
Steinbichler, T. B., Dudás, J., Skvortsov, S., Ganswindt, U., Riechelmann, H., & Skvortsova, I. I. (2018). Therapy resistance mediated by cancer stem cells. Seminars in Cancer Biology, 53, 156–167. https://doi.org/10.1016/j.semcancer.2018.11.006
Jaiswal, R., Luk, F., Dalla, P. V., Grau, G. E. R., & Bebawy, M. (2013). Breast cancer-derived microparticles display tissue selectivity in the transfer of resistance proteins to cells. PLoS ONE, 8, e61515.
Vesel, M., Rapp, J., Feller, D., Kiss, E., Jaromi, L., Meggyes, M., Miskei, G., Duga, B., Smuk, G., & Laszlo, T. (2017). ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respiratory Research, 18, 1–11.
Schinkel, A. H., Smit, J. J., van Tellingen, O., Beijnen, J. H., Wagenaar, E., van Deemter, L., Mol, C. A., van der Valk, M. A., Robanus-Maandag, E. C., te Riele, H. P., et al. (1994). Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell, 77, 491–502. https://doi.org/10.1016/0092-8674(94)90212-7
Johnson, W. W. (2002). P-glycoprotein-mediated efflux as a major factor in the variance of absorption and distribution of drugs: Modulation of chemotherapy resistance. Methods and Findings in Experimental and Clinical Pharmacology, 24, 501–514. https://doi.org/10.1358/mf.2002.24.8.705071
Cerezo, D., Lencina, M., Ruiz-Alcaraz, A. J., Ferragut, J. A., Saceda, M., Sanchez, M., Cánovas, M., García-Peñarrubia, P., & Martín-Orozco, E. (2012). Acquisition of MDR phenotype by leukemic cells is associated with increased caspase-3 activity and a collateral sensitivity to cold stress. Journal of Cellular Biochemistry, 113, 1416–1425. https://doi.org/10.1002/jcb.24016
Cerezo, D., Ruiz-Alcaraz, A. J., Lencina-Guardiola, M., Cánovas, M., García-Peñarrubia, P., Martínez-López, I., & Martín-Orozco, E. (2017). Attenuated JNK signaling in multidrug-resistant leukemic cells Dual role of MAPK in cell survival. Cell Signal, 30, 162–170. https://doi.org/10.1016/j.cellsig.2016.12.003
Pallis, M., & Russell, N. (2000). P-glycoprotein plays a drug-efflux-independent role in augmenting cell survival in acute myeloblastic leukemia and is associated with modulation of a sphingomyelin-ceramide apoptotic pathway. Blood, 95, 2897–2904.
Weisburg, J. H., Roepe, P. D., Dzekunov, S., & Scheinberg, D. A. (1999). Intracellular pH and multidrug resistance regulate complement-mediated cytotoxicity of nucleated human cells. Journal of Biological Chemistry, 274, 10877–10888. https://doi.org/10.1074/jbc.274.16.10877
Ding, S., Chamberlain, M., McLaren, A., Goh, L., Duncan, I., & Wolf, C. R. (2001). Cross-talk between signalling pathways and the multidrug resistant protein MDR-1. British Journal of Cancer, 85, 1175–1184. https://doi.org/10.1054/bjoc.2001.2044
Cerezo, D., Cánovas, M., García-Peñarrubia, P., & Martín-Orozco, E. (2015). Collateral sensitivity to cold stress and differential BCL-2 family expression in new daunomycin-resistant lymphoblastoid cell lines. Experimental Cell Research, 331, 11–20. https://doi.org/10.1016/j.yexcr.2014.11.017
Goler-Baron, V., & Assaraf, Y. G. (2011). Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS ONE, 6, e16007. https://doi.org/10.1371/journal.pone.0016007
Horio, M., Gottesman, M. M., & Pastan, I. (1988). ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proceedings of the National Academy of Sciences of the United States of America, 85, 3580–3584. https://doi.org/10.1073/pnas.85.10.3580
Leibovitz, A., Stinson, J. C., McCombs, W. B., 3rd., McCoy, C. E., Mazur, K. C., & Mabry Mabry, N. D. (1976). Classification of human colorectal adenocarcinoma cell lines. Cancer Research, 36, 4562–4569.
Xu, Z., Liu, Y., Yang, Y., Wang, J., Zhang, G., Liu1, Z., Fu, H., Wang, Z., Liu, H., & Xu, J. (2016). High expression of Mucin13 associates with grimmer postoperative prognosis of patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget, 8(5), 7548–7558.
Khan, S., Ebeling, M. C., Zaman, M. S., Sikander, M., Yallapu, M. M., Chauhan, N., Yacoubian, A. M., Behrman, S. W., Zafar, N., Kumar, D., et al. (2014). MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget, 5(17), 7599–7609.
Setua, S., Khan, S., Yallapu, M. M., Behrman, S. W., Sikander, M., Khan, S. S., Jaggi, M., & Chauhan, S. C. (2017). Restitution of tumor suppressor MicroRNA-145 using magnetic nanoformulation for pancreatic cancer therapy. Journal of Gastrointestinal Surgery, 21, 94–105. https://doi.org/10.1007/s11605-016-3222-z
Sikander, M. (2012). Mucins: Potential for ovarian cancer biomarkers. International Journal of Biomedical and Advance Research, 3(6). https://doi.org/10.7439/ijbar.v3i6.526.
Gupta, B. K., Sikander, M., & Jaggi, M. J. (2016). Gastroenterol Dig Dis 1(2). Link: Overview of mucin (MUC13) in gastrointestinal cancers. (https://www.alliedacademies.org/).
Sikander, M., Malik, S., Khan, S., Kumari, S., Chauhan, N., Khan, P., Halaweish, F.T., Chauhan, B., Yallapu, M. M., Jaggi, M., et al. (2019). Novel mechanistic insight into the anticancer activity of cucurbitacin D against pancreatic cancer (Cuc D attenuates pancreatic cancer). Cells, 9. https://doi.org/10.3390/cells9010103.
Mills, J. C., Stanger, B. Z., & Sander, M. (2019). Nomenclature for cellular plasticity: Are the terms as plastic as the cells themselves? EMBO Journal, 38, e103148. https://doi.org/10.15252/embj.2019103148
Yuan, S., Norgard, R. J., & Stanger, B. Z. (2019). Cellular plasticity in cancer. Cancer Discov, 9, 837–851. https://doi.org/10.1158/2159-8290.Cd-19-0015
Gurdon, J. B. (1962). The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Journal of Embryology and Experimental Morphology, 10, 622–640.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. https://doi.org/10.1016/j.cell.2006.07.024
Nieto, M. A., Huang, R. Y., Jackson, R. A., & Thiery, J. P. E. M. T. (2016). Cell, 2016(166), 21–45. https://doi.org/10.1016/j.cell.2016.06.028
Shibata, M., Hoque, M.O. (2019). Targeting cancer stem cells: A strategy for effective eradication of cancer. Cancers (Basel), 11 https://doi.org/10.3390/cancers11050732.
Khan, A.Q., Ahmed, E.I., Elareer, N. R., Junejo, K., Steinhoff, M., Uddin, S. (2019). Role of miRNA-regulated cancer stem cells in the pathogenesis of human malignancies. Cells, 8. https://doi.org/10.3390/cells8080840.
Iwata, M. (1968). Study of serum lipids in infants. 1. Serum lipids in healthy infants].Nihon Shonika Gakkai Zasshi, 72, 1075–1081.
Jordan, C. T., Guzman, M. L., & Noble, M. (2006). Cancer stem cells. New England Journal of Medicine, 355, 1253–1261. https://doi.org/10.1056/NEJMra061808
Thiery, J. P., Acloque, H., Huang, R. Y., & Nieto, M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139, 871–890. https://doi.org/10.1016/j.cell.2009.11.007
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., Brooks, M., Reinhard, F., Zhang, C. C., Shipitsin, M., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715. https://doi.org/10.1016/j.cell.2008.03.027
Polyak, K., & Weinberg, R. A. (2009). Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nature Reviews Cancer, 9, 265–273. https://doi.org/10.1038/nrc2620
Thiery, J. P. (2003). Epithelial-mesenchymal transitions in development and pathologies. Current Opinion in Cell Biology, 15, 740–746. https://doi.org/10.1016/j.ceb.2003.10.006
Huber, M. A., Kraut, N., & Beug, H. (2005). Molecular requirements for epithelial-mesenchymal transition during tumor progression. Current Opinion in Cell Biology, 17, 548–558. https://doi.org/10.1016/j.ceb.2005.08.001
Malanchi, I., Peinado, H., Kassen, D., Hussenet, T., Metzger, D., Chambon, P., Huber, M., Hohl, D., Cano, A., Birchmeier, W., et al. (2008). Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature, 452, 650–653. https://doi.org/10.1038/nature06835
Peacock, C. D., & Watkins, D. N. (2008). Cancer stem cells and the ontogeny of lung cancer. Journal of Clinical Oncology, 26, 2883–2889. https://doi.org/10.1200/jco.2007.15.2702
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 3983–3988. https://doi.org/10.1073/pnas.0530291100
Wielenga, V. J., Smits, R., Korinek, V., Smit, L., Kielman, M., Fodde, R., Clevers, H., & Pals, S. T. (1999). Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. American Journal of Pathology, 154, 515–523. https://doi.org/10.1016/s0002-9440(10)65297-2
Hermann, P. C., Huber, S. L., Herrler, T., Aicher, A., Ellwart, J. W., Guba, M., Bruns, C. J., & Heeschen, C. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 1, 313–323. https://doi.org/10.1016/j.stem.2007.06.002
Kumar, R., Gururaj, A. E., & Barnes, C. J. (2006). p21-activated kinases in cancer. Nature Reviews Cancer, 6, 459–471. https://doi.org/10.1038/nrc1892
Coniglio, S. J., Zavarella, S., & Symons, M. H. (2008). Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Molecular and Cellular Biology, 28, 4162–4172. https://doi.org/10.1128/mcb.01532-07
Boye, K., & Maelandsmo, G. M. (2010). S100A4 and metastasis: A small actor playing many roles. American Journal of Pathology, 176, 528–535. https://doi.org/10.2353/ajpath.2010.090526
Sherbet, G. V., & Lakshmi, M. S. (1998). S100A4 (MTS1) calcium binding protein in cancer growth, invasion and metastasis. Anticancer Research, 18, 2415–2421.
Feldner, J. C., & Brandt, B. H. (2002). Cancer cell motility–on the road from c-erbB-2 receptor steered signaling to actin reorganization. Experimental Cell Research, 272, 93–108. https://doi.org/10.1006/excr.2001.5385
Estrella, V., Chen, T., Lloyd, M., Wojtkowiak, J., Cornnell, H. H., Ibrahim-Hashim, A., Bailey, K., Balagurunathan, Y., Rothberg, J. M., Sloane, B. F., et al. (2013). Acidity generated by the tumor microenvironment drives local invasion. Cancer Research, 73, 1524–1535. https://doi.org/10.1158/0008-5472.Can-12-2796
Walenta, S., Wetterling, M., Lehrke, M., Schwickert, G., Sundfør, K., Rofstad, E. K., & Mueller-Klieser, W. (2000). High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Research, 60, 916–921.
Weinstein, I. B., & Joe, A. (2008). Oncogene addiction. Cancer Research, 68, 3077–3080. https://doi.org/10.1158/0008-5472.Can-07-3293
Sureshbabu, S. K., Chaukar, D., & Chiplunkar, S. V. (2020). Hypoxia regulates the differentiation and anti-tumor effector functions of γδT cells in oral cancer. Clinical and Experimental Immunology, 201, 40–57. https://doi.org/10.1111/cei.13436
Zhang, C., Samanta, D., Lu, H., Bullen, J. W., Zhang, H., Chen, I., He, X., & Semenza, G. L. (2016). Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proceedings of the National Academy of Sciences of the United States of America, 113, E2047-2056. https://doi.org/10.1073/pnas.1602883113
Cao, Z., Zheng, X., Yang, H., Li, S., Xu, F., Yang, X., & Wang, Y. (2020). Association of obesity status and metabolic syndrome with site-specific cancers: A population-based cohort study. British Journal of Cancer, 123, 1336–1344. https://doi.org/10.1038/s41416-020-1012-6
Ajduković, J. (2016). HIF-1–a big chapter in the cancer tale. Experimental Oncology, 38, 9–12.
Schito, L., & Semenza, G. L. (2016). Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer, 2, 758–770. https://doi.org/10.1016/j.trecan.2016.10.016
Mathieu, J., Zhang, Z., Zhou, W., Wang, A. J., Heddleston, J. M., Pinna, C. M., Hubaud, A., Stadler, B., Choi, M., Bar, M., et al. (2011). HIF induces human embryonic stem cell markers in cancer cells. Cancer Research, 71, 4640–4652. https://doi.org/10.1158/0008-5472.Can-10-3320
Lin, Y. T., & Wu, K. J. (2020). Epigenetic regulation of epithelial-mesenchymal transition: Focusing on hypoxia and TGF-β signaling. Journal of Biomedical Science, 27, 39. https://doi.org/10.1186/s12929-020-00632-3
Rankin, E. B., Nam, J. M., & Giaccia, A. J. (2016). Hypoxia: Signaling the metastatic cascade. Trends Cancer, 2, 295–304. https://doi.org/10.1016/j.trecan.2016.05.006
Hajizadeh, F., Okoye, I., Esmaily, M., Ghasemi Chaleshtari, M., Masjedi, A., Azizi, G., Irandoust, M., Ghalamfarsa, G., & Jadidi-Niaragh, F. (2019). Hypoxia inducible factors in the tumor microenvironment as therapeutic targets of cancer stem cells. Life Sciences, 237, 116952. https://doi.org/10.1016/j.lfs.2019.116952
Zhang, H., Lu, H., Xiang, L., Bullen, J. W., Zhang, C., Samanta, D., Gilkes, D. M., He, J., & Semenza, G. L. (2015). HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America, 112, E6215-6223. https://doi.org/10.1073/pnas.1520032112
Thomas, S., Harding, M. A., Smith, S. C., Overdevest, J. B., Nitz, M. D., Frierson, H. F., Tomlins, S. A., Kristiansen, G., & Theodorescu, D. (2012). CD24 is an effector of HIF-1-driven primary tumor growth and metastasis. Cancer Research, 72, 5600–5612. https://doi.org/10.1158/0008-5472.Can-11-3666
Ohnishi, S., Maehara, O., Nakagawa, K., Kameya, A., Otaki, K., Fujita, H., Higashi, R., Takagi, K., Asaka, M., Sakamoto, N., et al. (2013). hypoxia-inducible factors activate CD133 promoter through ETS family transcription factors. PLoS ONE, 8, e66255. https://doi.org/10.1371/journal.pone.0066255
Hashimoto, O., Shimizu, K., Semba, S., Chiba, S., Ku, Y., Yokozaki, H., & Hori, Y. (2011). Hypoxia induces tumor aggressiveness and the expansion of CD133-positive cells in a hypoxia-inducible factor-1α-dependent manner in pancreatic cancer cells. Pathobiology, 78, 181–192. https://doi.org/10.1159/000325538
Chiu, D. K., Zhang, M. S., Tse, A. P., & Wong, C. C. (2019). Assessment of stabilization and activity of the HIFs important for hypoxia-induced signalling in cancer cells. Methods in Molecular Biology, 1928, 77–99. https://doi.org/10.1007/978-1-4939-9027-6_6
Soeda, A., Park, M., Lee, D., Mintz, A., Androutsellis-Theotokis, A., McKay, R. D., Engh, J., Iwama, T., Kunisada, T., Kassam, A. B., et al. (2009). Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene, 28, 3949–3959. https://doi.org/10.1038/onc.2009.252
Xiang, L., & Semenza, G. L. (2019). Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy. Advances in Cancer Research, 141, 175–212. https://doi.org/10.1016/bs.acr.2018.11.001
Porta, C., Paglino, C., & Mosca, A. (2014). Targeting PI3K/Akt/mTOR signaling in cancer. Frontiers in Oncology, 4, 64. https://doi.org/10.3389/fonc.2014.00064
Li, H., Zeng, J., & Shen, K. (2014). PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Archives of Gynecology and Obstetrics, 290, 1067–1078. https://doi.org/10.1007/s00404-014-3377-3
Tapia, O., Riquelme, I., Leal, P., Sandoval, A., Aedo, S., Weber, H., Letelier, P., Bellolio, E., Villaseca, M., Garcia, P., et al. (2014). The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Archiv, 465, 25–33. https://doi.org/10.1007/s00428-014-1588-4
Honjo, S., Ajani, J. A., Scott, A. W., Chen, Q., Skinner, H. D., Stroehlein, J., Johnson, R. L., & Song, S. (2014). Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. International Journal of Oncology, 45, 567–574. https://doi.org/10.3892/ijo.2014.2450
Mohammed, A., Janakiram, N. B., Brewer, M., Ritchie, R. L., Marya, A., Lightfoot, S., Steele, V. E., & Rao, C. V. (2013). Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and mTOR signaling. Transl Oncol, 6, 649–659. https://doi.org/10.1593/tlo.13556
Chang, L., Graham, P. H., Hao, J., Ni, J., Bucci, J., Cozzi, P. J., Kearsley, J. H., & Li, Y. (2013). Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death & Disease, 4, e875. https://doi.org/10.1038/cddis.2013.407
Zhou, J., Wulfkuhle, J., Zhang, H., Gu, P., Yang, Y., Deng, J., Margolick, J. B., Liotta, L. A., Petricoin, E., 3rd., & Zhang, Y. (2007). Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proceedings of the National Academy of Sciences of the United States of America, 104, 16158–16163. https://doi.org/10.1073/pnas.0702596104
Douville, J., Beaulieu, R., & Balicki, D. (2009). ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev, 18, 17–25. https://doi.org/10.1089/scd.2008.0055
Huang, E. H., Hynes, M. J., Zhang, T., Ginestier, C., Dontu, G., Appelman, H., Fields, J. Z., Wicha, M. S., & Boman, B. M. (2009). Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Research, 69, 3382–3389. https://doi.org/10.1158/0008-5472.Can-08-4418
Nishitani, S., Horie, M., Ishizaki, S., & Yano, H. (2013). Branched chain amino acid suppresses hepatocellular cancer stem cells through the activation of mammalian target of rapamycin. PLoS ONE, 8, e82346. https://doi.org/10.1371/journal.pone.0082346
Sunayama, J., Matsuda, K., Sato, A., Tachibana, K., Suzuki, K., Narita, Y., Shibui, S., Sakurada, K., Kayama, T., Tomiyama, A., et al. (2010). Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells, 28, 1930–1939. https://doi.org/10.1002/stem.521
Bleau, A. M., Hambardzumyan, D., Ozawa, T., Fomchenko, E. I., Huse, J. T., Brennan, C. W., & Holland, E. C. (2009). PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell, 4, 226–235. https://doi.org/10.1016/j.stem.2009.01.007
Acknowledgements
This work was parcially supported by Start-up from Department of Immunology and Microbiology, School of Medicine, University of Texas Rio Grande Valley, and NIH grants (SC1GM139727, R01 CA210192, and R01 CA206069). Authors thank the CPRIT (RP210180 and RP230419) and UT-System Start Award facilities. Authors thank Ms. Anyssa Rodriguez and Ms. Molly K. Vela for proofreading this manuscript.
Author information
Authors and Affiliations
Contributions
Conceived the idea: S.M., M.S1. and S.C.C; Writing Original draft preparation: S.M., and M.S1; Supervision: S.C.C; Proofreading and editing: S.M, M.S1., S.C.C., S.K., A.D., M.M.Y., M.W., E.C., M.J., and M.S2. All authors have reviewed and given their approval to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shabnam Malik and Mohammed Sikander contributed equally to this manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malik, S., Sikander, M., Wahid, M. et al. Deciphering cellular and molecular mechanism of MUC13 mucin involved in cancer cell plasticity and drug resistance. Cancer Metastasis Rev (2024). https://doi.org/10.1007/s10555-024-10177-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10555-024-10177-8