Abstract
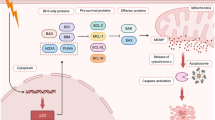
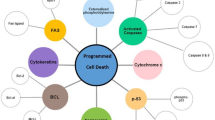
Tumor recurrence is a paradoxical function of a machinery, whereby a small proportion of the cancer cell population enters a resistant, dormant state, persists long-term in this condition, and then transitions to proliferation. The dormant phenotype is typical of cancer stem cells, tumor-initiating cells, disseminated tumor cells, and drug-tolerant persisters, which all demonstrate similar or even equivalent properties. Cancer cell dormancy and its conversion to repopulation are regulated by several protein signaling systems that inhibit or induce cell proliferation and provide optimal interrelations between cancer cells and their special niche; these systems act in close connection with tumor microenvironment and immune response mechanisms. During dormancy and reawakening periods, cell proteostasis machineries, autophagy, molecular chaperones, and the unfolded protein response are recruited to protect refractory tumor cells from a wide variety of stressors and therapeutic insults. Proteostasis mechanisms functionally or even physically interfere with the main regulators of tumor relapse, and the significance of these interactions and implications in the tumor recurrence phases are discussed in this review.





Similar content being viewed by others
References
Fluegen, G., Avivar-Valderas, A., Wang, Y., Padgen, M. R., Williams, J. K., Nobre, A. R., & Aguirre-Ghiso, J. A. (2017). Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol., 19(2), 120–132. https://doi.org/10.1038/ncb3465
De Angelis, M. L., Francescangeli, F., La Torre, F., & Zeuner, A. (2019). Stem cell plasticity and dormancy in the development of cancer therapy resistance. Front Oncol, 9, 626. https://doi.org/10.3389/fonc.2019.00626
Hen, O., & Barkan, D. (2020). Dormant disseminated tumor cells and cancer stem/progenitor-like cells: similarities and opportunities. Semin. Cancer Biol., 60, 157–165. https://doi.org/10.1016/j.semcancer.2019.09.002
De Angelis, M. L., Francescangeli, F., & Zeuner, A. (2019). Breast cancer stem cells as drivers of tumor chemoresistance, dormancy and relapse: new challenges and therapeutic opportunities. Cancers., 11(10), 1569. https://doi.org/10.3390/cancers11101569
Yang, C., Tian, G., Dajac, M., Doty, A., Wang, S., Lee, J. H., et al. (2022). Slow-cycling cells in glioblastoma: a specific population in the cellular mosaic of cancer stem cells. Cancers, 14(5), 1–18. https://doi.org/10.3390/cancers14051126
Nik Nabil, W. N., Xi, Z., Song, Z., Jin, L., Zhang, X. D., Zhou, H., et al. (2021). Towards a framework for better understanding of quiescent cancer cells. Cells., 10(3), 562. https://doi.org/10.3390/cells10030562
Basu, S., Dong, Y., Kumar, R., Jeter, C., & Tang, D. G. (2022). Slow-cycling (dormant) cancer cells in therapy resistance, cancer relapse and metastasis. Semin. Cancer Biol., 78, 90–103. https://doi.org/10.1016/j.semcancer.2021.04.021
Sistigu, A., Musella, M., Galassi, C., Vitale, I., & De Maria, R. (2020). Tuning cancer fate: tumor microenvironment’s role in cancer stem cell quiescence and reawakening. Front. Immunol., 11(October). https://doi.org/10.3389/fimmu.2020.02166
Tamamouna, V., Pavlou, E., Neophytou, C. M., Papageorgis, P., & Costeas, P. (2022). Regulation of metastatic tumor dormancy and emerging opportunities for therapeutic intervention. Int. J. Mol. Sci., 23(22), 13931. https://doi.org/10.3390/ijms232213931
Vera-Ramirez, L. (2020). Cell-intrinsic survival signals. The role of autophagy in metastatic dissemination and tumor cell dormancy. In Seminars in Cancer Biology (Vol. 60, pp. 28–40). Elsevier.
Yang, X., Liang, X., Zheng, M., & Tang, Y. (2018). Cellular phenotype plasticity in cancer dormancy and metastasis. Front Oncol, 8(NOV), 1–12. https://doi.org/10.3389/fonc.2018.00505
Damen, M. P. F., van Rheenen, J., & Scheele, C. L. G. J. (2021). Targeting dormant tumor cells to prevent cancer recurrence. FEBS Lett., 288(21), 6286–6303. https://doi.org/10.1111/febs.15626
Holmgren, L., O’reilly, M. S., & Folkman, J. (1995). Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med., 1(2), 149–153. https://doi.org/10.1038/nm0295-149
Racila, E., Scheuermann, R. H., Picker, L. J., Yefenof, E., Tucker, T., Chang, W., Marches, R., Street, N. E., & E. S. V. and J. W. hr. (1995). Tumor dormancy and cell signaling. II. Antibody as an agonist in inducing dormancy of a B cell lymphoma in SCID mice. J. Exp. Med., 181(April), 1539–1550. https://doi.org/10.1084/jem.181.4.1539
Zhou, N., Wu, X., Yang, B., Yang, X., Zhang, D., & Qing, G. (2014). Stem cell characteristics of dormant cells and cisplatin-induced effects on the stemness of epithelial ovarian cancer cells. Mol. Med. Rep., 10(5), 2495–2504. https://doi.org/10.3892/mmr.2014.2483
Carcereri de Prati, A., Butturini, E., Rigo, A., Oppici, E., Rossin, M., Boriero, D., & Mariotto, S. (2017). Metastatic breast cancer cells enter into dormant state and express cancer stem cells phenotype under chronic hypoxia. J. Cell. Biochem., 118(10), 3237–3248. https://doi.org/10.1002/jcb.25972
Hosseini, H., Obradovic, M. M. S., Hoffmann, M., Harper, K. L., Sosa, M. S., Werner-Klein, M., et al. (2016). Early dissemination seeds metastasis in breast cancer. Nature, 540(7634), 552–558. https://doi.org/10.1038/nature20785
Pommier, A., Anaparthy, N., Memos, N., Kelley, Z. L., Gouronnec, A., Yan, R., et al. (2018). Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science, 360(6394), eaao4908.
Baldominos, P., Barbera-Mourelle, A., Barreiro, O., Huang, Y., Wight, A., Cho, J.-W., et al. (2022). Quiescent cancer cells resist T cell attack by forming an immunosuppressive niche. Cell, 185(10), 1694–1708.e19. https://doi.org/10.1016/j.cell.2022.03.033
Peitzsch, C., Tyutyunnykova, A., Pantel, K., & Dubrovska, A. (2017). Cancer stem cells: the root of tumor recurrence and metastases. Semin. Cancer Biol., 44, 10–24. https://doi.org/10.1016/j.semcancer.2017.02.011
Xie, X. P., Laks, D. R., Sun, D., Ganbold, M., Wang, Z., Pedraza, A. M., et al. (2022). Quiescent human glioblastoma cancer stem cells drive tumor initiation, expansion, and recurrence following chemotherapy. Dev. Cell, 57(1), 32–46.e8. https://doi.org/10.1016/j.devcel.2021.12.007
Leonce, C., Saintigny, P., & Ortiz-Cuaran, S. (2022). Cell-intrinsic mechanisms of drug tolerance to systemic therapies in cancer. Mol. Cancer, 20(1), 11–29. https://doi.org/10.1158/1541-7786.MCR-21-0038
Dhanyamraju, P. K., Schell, T. D., Amin, S., & Robertson, G. P. (2022). Drug-tolerant persister cells in cancer therapy resistance. Cancer Res, 82(14), 2503–2514. https://doi.org/10.1158/0008-5472.CAN-21-3844
Delahaye, C., Figarol, S., Pradines, A., Favre, G., Mazieres, J., & Calvayrac, O. (2022). Early steps of resistance to targeted therapies in non-small-cell lung cancer. Cancers. https://doi.org/10.3390/cancers14112613
Santos-de-Frutos, K., & Djouder, N. (2021). When dormancy fuels tumour relapse. Commun. Biol., 4(1), 747. https://doi.org/10.1038/s42003-021-02257-0
Morales-Valencia, J., & David, G. (2022). The origins of cancer cell dormancy. Curr. Opin. Genet. Dev., 74, 101914. https://doi.org/10.1016/j.gde.2022.101914
Rehman, S. K., Haynes, J., Collignon, E., Brown, K. R., Wang, Y., Nixon, A. M. L., & Lo, E. B. L. (2021). Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. Cell, 184(1), 226–242.
Ramirez, M., Rajaram, S., Steininger, R. J., Osipchuk, D., Roth, M. A., Morinishi, L. S., & Altschuler, S. J. (2016). Diverse drug-resistance mechanisms can emerge from drug-tolerant cancer persister cells. Nat. Comm., 7, 1–8. https://doi.org/10.1038/ncomms10690
Oren, Y., Tsabar, M., Cuoco, M. S., Amir-Zilberstein, L., Cabanos, H. F., Hütter, J. C., et al. (2021). Cycling cancer persister cells arise from lineages with distinct programs. Nature, 596(7873), 576–582. https://doi.org/10.1038/s41586-021-03796-6
Russo, M., Pompei, S., Sogari, A., Corigliano, M., Crisafulli, G., Puliafito, A., & Cosentino Lagomarsino, M. (2022). A modified fluctuation-test framework characterizes the population dynamics and mutation rate of colorectal cancer persister cells. Nat. Gen., 54(7), 976–984. https://doi.org/10.1038/s41588-022-01105-z
Saleh, T., Tyutyunyk-Massey, L., & Gewirtz, D. A. (2019). Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Cancer Res., 79(6), 1044–1046. https://doi.org/10.1158/0008-5472.CAN-18-3437
Saleh, T., Bloukh, S., Carpenter, V. J., Alwohoush, E., Bakeer, J., Darwish, S., & Gewirtz, D. A. (2020). Therapy-induced senescence: an “old” friend becomes the enemy. Cancers., 12(4), 822. https://doi.org/10.3390/cancers12040822
Fitsiou, E., Soto-Gamez, A., & Demaria, M. (2022). Biological functions of therapy-induced senescence in cancer. Sem in Cancer Bio, 81, 5–13. https://doi.org/10.1016/j.semcancer.2021.03.021
Vallette, F. M., Olivier, C., Lézot, F., Oliver, L., Cochonneau, D., Lalier, L., et al. (2019). Dormant, quiescent, tolerant and persister cells: four synonyms for the same target in cancer. Bioc. Pharm., 162(September), 169–176. https://doi.org/10.1016/j.bcp.2018.11.004
Sauer, S., Reed, D. R., Ihnat, M., Hurst, R. E., Warshawsky, D., & Barkan, D. (2021). Innovative approaches in the battle against cancer recurrence: novel strategies to combat dormant disseminated tumor cells. Front in Onc., 11, 659963.
Risson, E., Nobre, A. R., Maguer-Satta, V., & Aguirre-Ghiso, J. A. (2020). The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer, 1(7), 672–680. https://doi.org/10.1038/s43018-020-0088-5
Li, X., Sun, Z., Peng, G., Xiao, Y., Guo, J., Wu, B., et al. (2022). Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics, 12(2), 620.
Lauber, K., & Herrmann, M. (2015). Tumor biology: with a little help from my dying friends. Curr. Bio., 25(5), R198–R201. https://doi.org/10.1016/j.cub.2015.01.040
Sulciner, M. L., Serhan, C. N., Gilligan, M. M., Mudge, D. K., Chang, J., Gartung, A., et al. (2018). Resolvins suppress tumor growth and enhance cancer therapy. J of Exp. Med, 215(1), 115–140. https://doi.org/10.1084/jem.20170681
Haak, V. M., Huang, S., & Panigrahy, D. (2021). Debris-stimulated tumor growth: a Pandora’s box? Cancer Metastasis Rev., 40(3), 791–801.
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D., & O. L. (2003). ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Urol. Oncol.: Semin. Orig., 63(1), 1684–1695.
Aguirre-Ghiso, J. A., Ossowski, L., & Rosenbaum, S. K. (2004). Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathwCancer Res.ay activation during primary and metastatic growth., 64(20), 7336–7345.
Gawrzak, S., Rinaldi, L., Gregorio, S., Arenas, E. J., Salvador, F., Urosevic, J., et al. (2018). MSK1 regulates luminal cell differentiation and metastatic dormancy in ER+ breast cancer. Nat. Cell Bio, 20(2), 211–221. https://doi.org/10.1038/s41556-017-0021-z
Aguirre-Ghiso, J. A., & Sosa, M. S. (2018). Emerging topics on disseminated cancer cell dormancy and the paradigm of metastasis. Annu. Rev. Cancer Biol., 2, 377–393.
Sosa, M. S., Bragado, P., & Aguirre-Ghiso, J. A. (2014). Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. rev. Cancer, 14(9), 611–622. https://doi.org/10.1038/nrc3793
Sosa, M. S., Parikh, F., Maia, A. G., Estrada, Y., Bosch, A., Bragado, P., et al. (2015). NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nat. Comm, 6, 1–14. https://doi.org/10.1038/ncomms7170
Borgen, E., Rypdal, M. C., Sosa, M. S., Renolen, A., Schlichting, E., Lønning, P. E., et al. (2018). NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Res, 20(1), 120. https://doi.org/10.1186/s13058-018-1049-0
Ren, G., Esposito, M., & Kang, Y. (2015). Bone metastasis and the metastatic niche. J of Mol. Med, 93(11), 1203–1212. https://doi.org/10.1007/s00109-015-1329-4
Celià-Terrassa, T., & Kang, Y. (2018). Metastatic niche functions and therapeutic opportunities. Nat. Cell Bio., 20(8), 868–877. https://doi.org/10.1038/s41556-018-0145-9
Bartosh, T. J., Ullah, M., Zeitouni, S., Beaver, J., & Prockop, D. J. (2016). Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc. Natl. Acad. Sci. U.S.A, 113(42), E6447–E6456. https://doi.org/10.1073/pnas.1612290113
Bliss, S. A., Sinha, G., Sandiford, O. A., Williams, L. M., Engelberth, D. J., Guiro, K., et al. (2016). Mesenchymal stem cell–derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res., 76(19), 5832–5844. https://doi.org/10.1158/0008-5472.CAN-16-1092
Lim, A. R., & Ghajar, C. M. (2022). Thorny ground, rocky soil: Tissue-specific mechanisms of tumor dormancy and relapse. Sem. in Cancer Bio, 78, 104–123. https://doi.org/10.1016/j.semcancer.2021.05.007
Sandiford, O. A., Donnelly, R. J., El-Far, M. H., Burgmeyer, L. M., Sinha, G., Pamarthi, S. H., et al. (2021). Mesenchymal stem cell–secreted extracellular vesicles instruct stepwise dedifferentiation of breast cancer cells into dormancy at the bone marrow perivascular region. Cancer Res., 81(6), 1567–1582. https://doi.org/10.1158/0008-5472.CAN-20-2434
Bragado, P., Estrada, Y., Parikh, F., Krause, S., Capobianco, C., Farina, H. G., & Aguirre-Ghiso, J. A. (2013). TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Bio., 15(11), 1351–1361. https://doi.org/10.1038/ncb2861
Nobre, A. R., Risson, E., Singh, D. K., Di Martino, J. S., Cheung, J. F., Wang, J., et al. (2021). Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGF-β2. Nat. Cancer, 2(3), 327–339. https://doi.org/10.1038/s43018-021-00179-8
Yu-Lee, L.-Y., Guoyu, Y., Lee, Y.-C., Lin, S.-C., Pan, J., Pan, T., Kai-Jie, Y., Liu, B., Creighton, C. J., Rodriguez-Canales, J., Villalobos, P. A., Wistuba, I. I., de Nadal, E., Posas, F., Gallick, G. E., & S.-H. L. (2018). Osteoblast-secreted factors mediate dormancy of metastatic prostate cancer in the bone via activation of the TGFβRIII- p38MAPK-pS249/T252RB pathway. Cancer Res., 78(11), 2911–2924. https://doi.org/10.1158/0008-5472.CAN-17-1051
Kobayashi, A., Okuda, H., Xing, F., Pandey, P. R., Watabe, M., Hirota, S., et al. (2011). Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J of Exp Med, 208(13), 2641–2655. https://doi.org/10.1084/jem.20110840
Sharma, S., Xing, F., Liu, Y., Wu, K., Said, N., Pochampally, R., et al. (2016). Secreted protein acidic and rich in cysteine (sparc) mediates metastatic dormancy of prostate cancer in bone. J of Biol Chem, 291(37), 19351–19363. https://doi.org/10.1074/jbc.M116.737379
Ghiso, J. A. A. (2002). Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene, 610(January). https://doi.org/10.1038/sj/onc/1205342
Aguirre-Ghiso, J. A., Liu, D., Mignatti, A., Kovalski, K., & Ossowski, L. (2001). Urokinase receptor and fibronectin regulate the ERKMAPK to p38MAPK activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Bio of the Cell, 12(4), 863–879. https://doi.org/10.1091/mbc.12.4.863
Shibue, T., & Weinberg, R. A. (2009). Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. U.S.A, 106(25), 10290–10295. https://doi.org/10.1073/pnas.0904227106
Ohta, Y., Fujii, M., Takahashi, S., Takano, A., Nanki, K., Matano, M., et al. (2022). Cell–matrix interface regulates dormancy in human colon cancer stem cells. Nature, 608(7924), 784–794. https://doi.org/10.1038/s41586-022-05043-y
Lee, L. H., Davis, L., Ylagan, L., Omilian, A. R., Attwood, K., Firat, C., et al. (2022). Identification of a subset of stage I colorectal cancer patients with high recurrence risk. J. Natl. Cancer Inst., 114(5), 732–739.
Touil, Y., Igoudjil, W., Corvaisier, M., Dessein, A. F., Vandomme, J., Monte, D., & Huet, G. (2014). Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin. Cancer Res., 20(4), 837–846. https://doi.org/10.1158/1078-0432.CCR-13-1854
Kurppa, K. J., Liu, Y., To C, Zhang, T., Fan, M., Vajdi, A., Knelson, E. H., Xie, Y., Lim, K., Cejas, P., & Portell, A. (2020). Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer cell, 37(1), 104–122.
Francescangeli, F., Contavalli, P., De Angelis, M. L., Careccia, S., Signore, M., Haas, T. L., & Zeuner, A. (2020). A pre-existing population of ZEB2+ quiescent cells with stemness and mesenchymal features dictate chemoresistance in colorectal cancer. J. Exp. Clin. Cancer Res., 39(1), 2. https://doi.org/10.1186/s13046-019-1505-4
Cuccu, A., Francescangeli, F., De Angelis, M. L., Bruselles, A., Giuliani, A., & Zeuner, A. (2022). Analysis of dormancy-associated transcriptional networks reveals a shared quiescence signature in lung and colorectal cancer. Int. J. Mol. Sci., 23(17), 9869. https://doi.org/10.3390/ijms23179869
Müller, A., Homey, B., Soto, H., Ge, N., Catron, D., Buchanan, M. E., et al. (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature, 410(6824), 50–56. https://doi.org/10.1038/35065016
Gao, X. L., Zheng, M., Wang, H. F., Dai, L. L., Yu, X. H., Yang, X., & Tang, Y. L. (2019). NR2F1 contributes to cancer cell dormancy, invasion and metastasis of salivary adenoid cystic carcinoma by activating CXCL12/CXCR4 pathway. BMC Cancer, 19(1), 1–12. https://doi.org/10.1186/s12885-019-5925-5
Agarwal, P., Isringhausen, S., Li, H., Paterson, A. J., He, J., Gomariz, Á., et al. (2019). Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell, 24(5), 769–784.e6. https://doi.org/10.1016/j.stem.2019.02.018
Nielsen, S. R., Quaranta, V., Linford, A., Emeagi, P., Rainer, C., Santos, A., et al. (2016). Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat. Cell Bio., 18(5), 549–560. https://doi.org/10.1038/ncb3340
Krall, J. A., Reinhardt, F., Mercury, O. A., Pattabiraman, D. R., Brooks, M. W., Dougan, M., et al. (2018). The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Trans Med., 10(436), 1–12. https://doi.org/10.1126/scitranslmed.aan3464
Manjili, S. H., Isbell, M., Ghochaghi, N., Perkinson, T., & Manjili, M. H. (2022). Multifaceted functions of chronic inflammation in regulating tumor dormancy and relapse. Sem in Cancer Bio, 78(March), 17–22. https://doi.org/10.1016/j.semcancer.2021.03.023
Cackowski, F. C., Eber, M. R., Rhee, J., Decker, A. M., Yumoto, K., Berry, J. E., et al. (2017). Mer tyrosine kinase regulates disseminated prostate cancer cellular dormancy. J of cellular biochem., 118(4), 891–902.
Ruppender, N., Larson, S., Lakely, B., Kollath, L., Brown, L., Coleman, I., et al. (2015). Cellular adhesion promotes prostate cancer cells escape from dormancy. PLoS ONE, 10(6), 1–16. https://doi.org/10.1371/journal.pone.0130565
El Touny, L. H., Vieira, A., Mendoza, A., Khanna, C., Hoenerhoff, M. J., & Green, J. E. (2014). Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J. Clin. Investig., 124(1), 156–168. https://doi.org/10.1172/JCI70259
Simpkins, F., Jang, K., Yoon, H., Hew, K. E., Kim, M., Azzam, D. J., & Slingerland, J. M. (2018). Dual Src and MEK inhibition decreases ovarian cancer growth and targets tumor initiating stem-like cells. Clin. Cancer Res., 24(19), 4874–4886. https://doi.org/10.1158/1078-0432.CCR-17-3697
Kesh, K., Gupta, V. K., Durden, B., Garrido, V., Mateo-Victoriano, B., Lavania, S. P., & Banerjee, S. (2020). Therapy resistance, cancer stem cells and ECM in cancer: the matrix reloaded. Cancers, 12(10), 1–17. https://doi.org/10.3390/cancers12103067
Lorusso, G., Rüegg, C., & Kuonen, F. (2020). Targeting the extra-cellular matrix—tumor cell crosstalk for anti-cancer therapy: emerging alternatives to integrin inhibitors. Front. in Onc, 10(July), 1–17. https://doi.org/10.3389/fonc.2020.01231
Harrison, D. A. (2012). The jak/stat pathway. Cold Spring Harb. Perspect. Biol., 4(3), a011205.
Hu, M. T., Wang, J. H., Yu, Y., Liu, C., Li, B., Cheng, Q. B., & Jiang, X. Q. (2018). Tumor suppressor LKB1 inhibits the progression of gallbladder carcinoma and predicts the prognosis of patients with this malignancy. Int. J. Oncol., 53(3), 1215–1226. https://doi.org/10.3892/ijo.2018.4466
Teng, Y., Wang, X., Wang, Y., & Ma, D. (2010). Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem. Biophys. Res. Commun., 392(3), 373–379. https://doi.org/10.1016/j.bbrc.2010.01.028
Matsuoka, K., Bakiri, L., Wolff, L. I., Linder, M., Mikels-Vigdal, A., Patiño-García, A., et al. (2020). Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res., 30(10), 885–901. https://doi.org/10.1038/s41422-020-0370-1
Shah, D., Wyatt, D., Baker, A. T., Simms, P., Peiffer, D. S., Fernandez, M., et al. (2018). Inhibition of her2 increases jagged1-dependent breast cancer stem cells: role for membrane jagged1. Cli. Cancer Res., 24(18), 4566–4578. https://doi.org/10.1158/1078-0432.CCR-17-1952
Indraccolo, S., Minuzzo, S., Masiero, M., Pusceddu, I., Persano, L., Moserle, L., et al. (2009). Cross-talk between tumor and endothelial cells involving the Notch3-Dll4 interaction marks escape from tumor dormancy. Cancer Res., 69(4), 1314–1323. https://doi.org/10.1158/0008-5472.CAN-08-2791
Lawson, D. A., Bhakta, N. R., Kessenbrock, K., Prummel, K. D., Yu, Y., Takai, K., et al. (2015). Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature, 526(7571), 131–135. https://doi.org/10.1038/nature15260
Lu, X., Mu, E., Wei, Y., Riethdorf, S., Yang, Q., Yuan, M., et al. (2011). VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell, 20(6), 701–714. https://doi.org/10.1016/j.ccr.2011.11.002
Dai, R., Liu, M., Xiang, X., Xi, Z., & Xu, H. (2022). Osteoblasts and osteoclasts: an important switch of tumour cell dormancy during bone metastasis. J. Exp. Clin. Cancer Res., 41(1), 316. https://doi.org/10.1186/s13046-022-02520-0
Albrengues, J., Shields, M. A., Ng, D., Park, C. G., Ambrico, A., Poindexter, M. E., et al. (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science, 361(6409), eaao4227. https://doi.org/10.1126/science.aao4227
Xue, R., Zhang, Q., Cao, Q., Kong, R., Xiang, X., Liu, H., et al. (2022). Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature, 612(7938), 141–147. https://doi.org/10.1038/s41586-022-05400-x
Zhang, C., Li, D., Yu, R., Li, C., Song, Y., Chen, X., & Qu, X. (2021). Immune landscape of gastric carcinoma tumor microenvironment identifies a peritoneal relapse relevant immune signature. Front. Immunol., 12, 651033. https://doi.org/10.3389/fimmu.2021.651033
Ombrato, L., & Montagner, M. (2020). Technical advancements for studying immune regulation of disseminated dormant cancer cells. Front Oncol, 10, 594514. https://doi.org/10.3389/fonc.2020.594514
Chen, K., Zhang, C., Ling, S., Wei, R., Wang, J., & Xu, X. (2021). The metabolic flexibility of quiescent CSC: implications for chemotherapy resistance. Cell Death Dis., 12(9), 835. https://doi.org/10.1038/s41419-021-04116-6
Heft Neal, M. E., Brenner, J. C., Prince, M. E. P., & Chinn, S. B. (2022). Advancement in cancer stem cell biology and precision medicine—review article head and neck cancer stem cell plasticity and the tumor microenvironment. Front. Cell Dev. Biol., 9. https://doi.org/10.3389/fcell.2021.660210
Phan, T. G., & Croucher, P. I. (2020). The dormant cancer cell life cycle. Nat. Rev. Cancer, 20(7), 398–411. https://doi.org/10.1038/s41568-020-0263-0
Aguirre-Ghiso, J. A. (2021). Translating the science of cancer dormancy to the clinic. Cancer Res., 81(18), 4673–4675. https://doi.org/10.1158/0008-5472.CAN-21-1407
Gao, H., Chakraborty, G., Lee-Lim, A. P., Mo, Q., Decker, M., Vonica, A., et al. (2012). The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell, 150(4), 764–779. https://doi.org/10.1016/j.cell.2012.06.035
Ghajar, C. M., Peinado, H., Mori, H., Matei, I. R., Evason, K. J., Brazier, H., & Stainier, D. Y. R. (2013). The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol., 15(7), 807–817.
Roberts, D. D. (2008). Thrombospondins: from structure to therapeutics. Cell. Mol. Life Sci., 65(5), 669.
Lu, Z., Luo, R. Z., Lu, Y., Zhang, X., Yu, Q., Khare, S., et al. (2008). The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Investig., 118(12), 3917–3929. https://doi.org/10.1172/JCI35512
Mao, W., Peters, H. L., Sutton, M. N., Orozco, A. F., Pang, L., Yang, H., et al. (2019). The role of vascular endothelial growth factor, interleukin 8, and insulinlike growth factor in sustaining autophagic DIRAS3-induced dormant ovarian cancer xenografts. Cancer, 125(8), 1267–1280.
Sutton, M. N., Lu, Z., Li, Y.-C., Zhou, Y., Huang, T., Reger, A. S., et al. (2019). DIRAS3 (ARHI) blocks RAS/MAPK signaling by binding directly to RAS and disrupting RAS clusters. Cell rep, 29(11), 3448–3459.
Barkan, D., El Touny, L. H., Michalowski, A. M., Smith, J. A., Chu, I., Davis, A. S., & Gauldie, J. (2010). Metastatic growth from dormant cells induced by a Col-I–enriched fibrotic environmentmetastatic outgrowth from dormant tumor cells. Cancer res., 70(14), 5706–5716.
Du, C., Zheng, Z., Li, D., Chen, L., Li, N., Yi, X., & Xie, X. (2016). BKCa promotes growth and metastasis of prostate cancer through facilitating the coupling between αvβ3 integrin and FAK. Oncotarget, 7(26), 40174.
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D., & Ossowski, L. (2003). ERKMAPK activity as a determinant of tumor growth and dormancy; regulation by p38SAPK. Cancer res., 63(7), 1684–1695.
Park, S.-Y., & Nam, J.-S. (2020). The force awakens: metastatic dormant cancer cells. Exp. Mol. Med., 52(4), 569–581. https://doi.org/10.1038/s12276-020-0423-z
Correa, R. J. M., Peart, T., Valdes, Y. R., DiMattia, G. E., & Shepherd, T. G. (2012). Modulation of AKT activity is associated with reversible dormancy in ascites-derived epithelial ovarian cancer spheroids. Carcinogenesis, 33(1), 49–58. https://doi.org/10.1093/carcin/bgr241
Yang, L., He, C., Chen, X., Su, L., Liu, B., & Zhang, H. (2016). Aurora kinase A revives dormant laryngeal squamous cell carcinoma cells via FAK/PI3K/Akt pathway activation. Oncotarget, 7(30), 48346.
Klionsky, D. J., Petroni, G., Amaravadi, R. K., Baehrecke, E. H., Ballabio, A., Boya, P., et al. (2021). Autophagy in major human diseases. The EMBO j., 40(19), e108863. https://doi.org/10.15252/embj.2021108863
Panda, P. K., Mukhopadhyay, S., Das, D. N., Sinha, N., Naik, P. P., & Bhutia, S. K. (2015). Mechanism of autophagic regulation in carcinogenesis and cancer therapeutics. Semin. Cell Dev. Biol., 39, 43–55. https://doi.org/10.1016/j.semcdb.2015.02.013
Chavez-Dominguez, R., Perez-Medina, M., Lopez-Gonzalez, J. S., Galicia-Velasco, M., & Aguilar-Cazares, D. (2020). The double-edge sword of autophagy in cancer: from tumor suppression to pro-tumor activity. Front. Oncol., 10, 578418. https://doi.org/10.3389/fonc.2020.578418
Coto-Llerena, M., Tosti, N., Taha-Mehlitz, S., Kancherla, V., Paradiso, V., Gallon, J., et al. (2021). Transcriptional enhancer factor domain family member 4 exerts an oncogenic role in hepatocellular carcinoma by hippo-independent regulation of heat shock protein 70 family members. Hepatol. Commun., 5(4), 661–674. https://doi.org/10.1002/hep4.1656
Takamura, A., Komatsu, M., Hara, T., Sakamoto, A., Kishi, C., Waguri, S., et al. (2011). Autophagy-deficient mice develop multiple liver tumors. Genes & dev., 25(8), 795–800.
Wei, H., Wei, S., Gan, B., Peng, X., Zou, W., & Guan, J.-L. (2011). Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes & dev., 25(14), 1510–1527.
Ye, C., Yu, X., Liu, X., Zhan, P., Nie, T., Guo, R., et al. (2018). Beclin-1 knockdown decreases proliferation, invasion and migration of Ewing sarcoma SK-ES-1 cells via inhibition of MMP-9 Corrigendum in/10.3892/ol. 2020.12372. Onco. Lett., 15(3), 3221–3225.
Liu, M., Jiang, L., Fu, X., Wang, W., Ma, J., Tian, T., et al. (2018). Cytoplasmic liver kinase B1 promotes the growth of human lung adenocarcinoma by enhancing autophagy. Cancer sci., 109(10), 3055–3067.
Yun, C. W., & Lee, S. H. (2018). The roles of autophagy in cancer. Int. J. Mol. Sci., 19(11), 3466.
Guo, J. Y., Teng, X., Laddha, S. V., Ma, S., Van Nostrand, S. C., Yang, Y., et al. (2016). Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes & dev., 30(15), 1704–1717.
Xie, X., Koh, J. Y., Price, S., White, E., & Mehnert, J. M. (2015). Atg7 overcomes senescence and promotes growth of Braf V600E-driven melanoma. Cancer disc., 5(4), 410–423.
Strohecker, A. M., Guo, J. Y., Karsli-Uzunbas, G., Price, S. M., Chen, G. J., Mathew, R., et al. (2013). Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E–driven lung tumorsautophagy promotes BrafV600E-driven lung tumor growth. Cancer disc., 3(11), 1272–1285.
Lopiccolo, J., Kawabata, S., Gills, J. J., & Dennis, P. A. (2021). Combining nelfinavir with chloroquine inhibits in vivo growth of human lung cancer xenograft tumors. in vivo, 35(1), 141–145.
Yang, S., Ren, X., Liang, Y., Yan, Y., Zhou, Y., Hu, J., et al. (2020). KNK437 restricts the growth and metastasis of colorectal cancer via targeting DNAJA1/CDC45 axis. Oncogene, 39(2), 249–261. https://doi.org/10.1038/s41388-019-0978-0
Wang, F.-T., Wang, H., Wang, Q.-W., Pan, M.-S., Li, X.-P., Sun, W., & Fan, Y.-Z. (2020). Inhibition of autophagy by chloroquine enhances the antitumor activity of gemcitabine for gallbladder cancer. Cancer Chemother Pharmacol, 86(2), 221–232.
Chen, H., Lin, C., Lu, C., Wang, Y., Han, R., Li, L., & He, Y. (2019). Metformin-sensitized NSCLC cells to osimertinib via AMPK-dependent autophagy inhibition. Clin Respir J, 13(12), 781–790. https://doi.org/10.1111/crj.13091
Bi, Y., Jiang, Y., Li, X., Hou, G., & Li, K. (2021). Rapamycin inhibits lung squamous cell carcinoma growth by downregulating glypican-3/Wnt/β-catenin signaling and autophagy. J. Cancer Res. Clin. Oncol., 147(2), 499–505. https://doi.org/10.1007/s00432-020-03422-4
Das, C. K., Mandal, M., & Kögel, D. (2018). Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastasis Rev., 37(4), 749–766. https://doi.org/10.1007/s10555-018-9727-z
Bortnik, S., Tessier-Cloutier, B., Leung, S., Xu, J., Asleh, K., Burugu, S., et al. (2020). Differential expression and prognostic relevance of autophagy-related markers ATG4B, GABARAP, and LC3B in breast cancer. Breast Cancer Res. Treat., 183(3), 525–547.
Chen, J. L., David, J., Cook-Spaeth, D., Casey, S., Cohen, D., Selvendiran, K., et al. (2017). Autophagy induction results in enhanced anoikis resistance in models of peritoneal disease. Mol. Cancer Res., 15(1), 26–34.
Lu, J., Zhu, L., Zheng, L., Cui, Q., Zhu, H., Zhao, H., et al. (2018). Overexpression of ULK1 represents a potential diagnostic marker for clear cell renal carcinoma and the antitumor effects of SBI-0206965. EBioMedicine, 34, 85–93. https://doi.org/10.1016/j.ebiom.2018.07.034
Chen, Y., & Gibson, S. B. (2021). Three dimensions of autophagy in regulating tumor growth: cell survival/death, cell proliferation, and tumor dormancy. Biochim Biophys Acta Mol Basis Dis BBA-MOL BASIS DIS, 1867(12), 166265. https://doi.org/10.1016/j.bbadis.2021.166265
Smith, A. G., & Macleod, K. F. (2019). Autophagy, cancer stem cells and drug resistance. J Pathol, 247(5), 708–718. https://doi.org/10.1002/path.5222
Akkoc, Y., Peker, N., Akcay, A., & Gozuacik, D. (2021). Autophagy and cancer dormancy. Front Oncol, 11, 277. https://doi.org/10.3389/fonc.2021.627023
Mowers, E. E., Sharifi, M. N., & Macleod, K. F. (2018). Functions of autophagy in the tumor microenvironment and cancer metastasis. The FEBS J., 285(10), 1751–1766. https://doi.org/10.1111/febs.14388
Kimmelman, A. C., & White, E. (2017). Autophagy and tumor metabolism. Cell metabolism, 25(5), 1037–1043.
Bellot, G., Garcia-Medina, R., Gounon, P., Chiche, J., Roux, D., Pouysségur, J., & Mazure, N. M. (2009). Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol., 29(10), 2570–2581.
Hu, Y.-L., DeLay, M., Jahangiri, A., Molinaro, A. M., Rose, S. D., Carbonell, W. S., & Aghi, M. K. (2012). Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastomaautophagy mediates resistance to antiangiogenic therapy. Cancer res., 72(7), 1773–1783.
Yu, Y., Liu, B., Li, X., Lu, D., Yang, L., Chen, L., et al. (2022). ATF4/CEMIP/PKCα promotes anoikis resistance by enhancing protective autophagy in prostate cancer cells. Cell death & dis., 13(1), 1–13.
Avivar-Valderas, A., Salas, E., Bobrovnikova-Marjon, E., Diehl, J. A., Nagi, C., Debnath, J., & Aguirre-Ghiso, J. A. (2011). PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol. Cell. Biol., 31(17), 3616–3629.
Avivar-Valderas, A., Bobrovnikova-Marjon, E., Alan Diehl, J., Bardeesy, N., Debnath, J., & Aguirre-Ghiso, J. A. (2013). Regulation of autophagy during ECM detachment is linked to a selective inhibition of mTORC1 by PERK. Oncogene, 32(41), 4932–4940.
Fu, X.-T., Shi, Y.-H., Zhou, J., Peng, Y.-F., Liu, W.-R., Shi, G.-M., et al. (2018). MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer lett., 412, 108–117.
Peng, Y.-F., Shi, Y.-H., Ding, Z.-B., Ke, A.-W., Gu, C.-Y., Hui, B., et al. (2013). Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy, 9(12), 2056–2068.
Sandilands, E., Schoenherr, C., & Frame, M. C. (2015). p70S6K is regulated by focal adhesion kinase and is required for Src-selective autophagy. Cellular sig., 27(9), 1816–1823.
Cheng, Z., Zhu, Q., Dee, R., Opheim, Z., Mack, C. P., Cyr, D. M., & Taylor, J. M. (2017). Focal adhesion kinase-mediated phosphorylation of Beclin1 protein suppresses cardiomyocyte autophagy and initiates hypertrophic growth. J. Biol. Chem., 292(6), 2065–2079. https://doi.org/10.1074/jbc.M116.758268
Zhao, M., Finlay, D., Kwong, E., Liddington, R., Viollet, B., Sasaoka, N., & Vuori, K. (2022). Cell adhesion suppresses autophagy via Src/FAK-mediated phosphorylation and inhibition of AMPK. Cell. Signal, 89, 110170. https://doi.org/10.1016/j.cellsig.2021.110170
Zhao, M., Finlay, D., Liddington, R., & Vuori, K. (2022). SRC plays a specific role in the cross-talk between apoptosis and autophagy via phosphorylation of a novel regulatory site on AMPK. Autophagy Reports, 1(1), 38–41. https://doi.org/10.1080/27694127.2022.2047266
Abbi, S., Ueda, H., Zheng, C., Cooper, L. A., Zhao, J., Christopher, R., & Guan, J.-L. (2002). Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol. Biol. Cell., 13(9), 3178–3191. https://doi.org/10.1091/mbc.e02-05-0295
Sandilands, E., Serrels, B., McEwan, D. G., Morton, J. P., Macagno, J. P., McLeod, K., et al. (2012). Autophagic targeting of Src promotes cancer cell survival following reduced FAK signalling. Nat. Cell Biol., 14(1), 51–60. https://doi.org/10.1038/ncb2386
Schoenherr, C., Byron, A., Sandilands, E., Paliashvili, K., Baillie, G. S., Garcia-Munoz, A., et al. (2017). Ambra1 spatially regulates Src activity and Src/FAK-mediated cancer cell invasion via trafficking networks. eLife, 6, e23172. https://doi.org/10.7554/eLife.23172
Song, Q., Mao, B., Cheng, J., Gao, Y., Jiang, K., Chen, J., et al. (2015). YAP enhances autophagic flux to promote breast cancer cell survival in response to nutrient deprivation. PLOS ONE, 10(3), e0120790.
Pavel, M., Renna, M., Park, S. J., Menzies, F. M., Ricketts, T., Füllgrabe, J., et al. (2018). Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun., 9(1), 2961. https://doi.org/10.1038/s41467-018-05388-x
Totaro, A., Zhuang, Q., Panciera, T., Battilana, G., Azzolin, L., Brumana, G., et al. (2019). Cell phenotypic plasticity requires autophagic flux driven by YAP/TAZ mechanotransduction. Proc. Natl. Acad. Sci. U.S.A., 116(36), 17848–17857. https://doi.org/10.1073/pnas.1908228116
Jiang, Y., Ji, F., Liu, Y., He, M., Zhang, Z., Yang, J., et al. (2017). Cisplatin-induced autophagy protects breast cancer cells from apoptosis by regulating yes-associated protein. Oncol Rep, 38(6), 3668–3676. https://doi.org/10.3892/or.2017.6035
Xiao, L., Shi, X.-Y., Zhang, Y., Zhu, Y., Zhu, L., Tian, W., et al. (2016). YAP induces cisplatin resistance through activation of autophagy in human ovarian carcinoma cells. OncoTargets Ther., 9, 1105.
Chen, W., Bai, Y., Patel, C., & Geng, F. (2019). Autophagy promotes triple negative breast cancer metastasis via YAP nuclear localization. Biochem. Biophys. Res. Commun., 520(2), 263–268. https://doi.org/10.1016/j.bbrc.2019.09.133
Park, H. S., Lee, D.-H., Kang, D. H., Yeo, M.-K., Bae, G., Lee, D., et al. (2021). Targeting YAP-p62 signaling axis suppresses the EGFR-TKI-resistant lung adenocarcinoma. Cancer Med., 10(4), 1405–1417. https://doi.org/10.1002/cam4.3734
Zhao, M., Zhang, Y., Jiang, Y., Wang, K., Wang, X., Zhou, D., et al. (2021). YAP promotes autophagy and progression of gliomas via upregulating HMGB1. J. Exp. Clin. Cancer Res., 40(1), 99. https://doi.org/10.1186/s13046-021-01897-8
Tong, H., Yin, H., Hossain, M. A., Wang, Y., Wu, F., Dong, X., et al. (2019). Starvation-induced autophagy promotes the invasion and migration of human bladder cancer cells via TGF-β1/Smad3-mediated epithelial-mesenchymal transition activation. J. Cell. Biochem., 120(4), 5118–5127. https://doi.org/10.1002/jcb.27788
Li, J., Yang, B., Zhou, Q., Wu, Y., Shang, D., Guo, Y., et al. (2013). Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transition. Carcinogenesis, 34(6), 1343–1351. https://doi.org/10.1093/carcin/bgt063
Hussein, M. R. (2005). Transforming growth factor-β and malignant melanoma: molecular mechanisms. J. Cutan. Pathol., 32(6), 389–395. https://doi.org/10.1111/j.0303-6987.2005.00356.x
Sosa, M. S., Bragado, P., Debnath, J., & Aguirre-Ghiso, J. A. (2013). In H. Enderling, N. Almog, & L. Hlatky (Eds.), Regulation of tumor cell dormancy by tissue microenvironments and autophagy BT - systems biology of tumor dormancy (pp. 73–89). New York, NY, Springer New York.
Dash, S., Sarashetti, P. M., Rajashekar, B., Chowdhury, R., & Mukherjee, S. (2018). TGF-β2-induced EMT is dampened by inhibition of autophagy and TNF-α treatment. Oncotarget, 9(5), 6433.
Alizadeh, J., Glogowska, A., Thliveris, J., Kalantari, F., Shojaei, S., Hombach-Klonisch, S., et al. (2018). Autophagy modulates transforming growth factor beta 1 induced epithelial to mesenchymal transition in non-small cell lung cancer cells. Biochimica et Biophysica Acta (BBA) - Molecular. Cell Res., 1865(5), 749–768. https://doi.org/10.1016/j.bbamcr.2018.02.007
Trelford, C. B., & Di Guglielmo, G. M. (2022). Autophagy regulates transforming growth factor β signaling and receptor trafficking. Biochimica et Biophysica Acta (BBA) - Molecular. Cell Res., 1869(9), 119284. https://doi.org/10.1016/j.bbamcr.2022.119284
Yeo, S. K., Wen, J., Chen, S., & Guan, J.-L. (2016). Autophagy differentially regulates distinct breast cancer stem-like cells in murine models via EGFR/Stat3 and Tgfβ/Smad signalingregulation of distinct breast cancer stem cells by autophagy. Cancer res., 76(11), 3397–3410.
Pribluda, A., de la Cruz, C. C., & Jackson, E. L. (2015). Intratumoral heterogeneity: from diversity comes resistance. Cli. Cancer Res., 21(13), 2916–2923. https://doi.org/10.1158/1078-0432.CCR-14-1213
De Conti, G., Dias, M. H., & Bernards, R. (2021). Fighting drug resistance through the targeting of drug-tolerant persister cells. Cancers. https://doi.org/10.3390/cancers13051118
Gupta, A., Roy, S., Lazar, A. J. F., Wang, W.-L., McAuliffe, J. C., Reynoso, D., et al. (2010). Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST). Proc. Natl. Acad. Sci. U.S.A., 201000248. https://doi.org/10.1073/pnas.1000248107
Viale, A., Pettazzoni, P., Lyssiotis, C. A., Ying, H., Sánchez, N., Marchesini, M., et al. (2014). Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature, 514(7524), 628–632. https://doi.org/10.1038/nature13611
Yu, Z., Zhou, R., Zhao, Y., Pan, Y., Liang, H., Zhang, J.-S., et al. (2019). Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell Prolif., 52(2), e12568. https://doi.org/10.1111/cpr.12568
Shimizu, T., Sugihara, E., Yamaguchi-Iwai, S., Tamaki, S., Koyama, Y., Kamel, W., et al. (2014). IGF2 preserves osteosarcoma cell survival by creating an autophagic state of Dormancy That Protects Cells against Chemotherapeutic StressIGF/insulin signaling induces dormancy in osteosarcoma. Cancer res., 74(22), 6531–6541.
Correa, R. J. M., Valdes, Y. R., Peart, T. M., Fazio, E. N., Bertrand, M., McGee, J., et al. (2014). Combination of AKT inhibition with autophagy blockade effectively reduces ascites-derived ovarian cancer cell viability. Carcinogenesis, 35(9), 1951–1961. https://doi.org/10.1093/carcin/bgu049
Kim, J. K., Jung, Y., Wang, J., Joseph, J., Mishra, A., Hill, E. E., et al. (2013). TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia, 15(9), 1064–1074. https://doi.org/10.1593/neo.13402
Lu, Z., Yang, H., Sutton, M. N., Yang, M., Clarke, C. H., Liao, W. S. L., & Bast, R. C. (2014). ARHI (DIRAS3) induces autophagy in ovarian cancer cells by downregulating the epidermal growth factor receptor, inhibiting PI3K and Ras/MAP signaling and activating the FOXo3a-mediated induction of Rab7. Cell Death Differ, 21(8), 1275–1289.
You, B., Xia, T., Gu, M., Zhang, Z., Zhang, Q., Shen, J., et al. (2022). AMPK-mTOR-mediated activation of autophagy promotes formation of dormant polyploid giant cancer cells. Cancer Res, 82(5), 846–858. https://doi.org/10.1158/0008-5472.CAN-21-2342
Lu, Z., Baquero, M. T., Yang, H., Yang, M., Reger, A. S., Kim, C., et al. (2014). DIRAS3 regulates the autophagosome initiation complex in dormant ovarian cancer cells. Autophagy, 10(6), 1071–1092. https://doi.org/10.4161/auto.28577
Esposito, A., Ferraresi, A., Salwa, A., Vidoni, C., Dhanasekaran, D. N., & Isidoro, C. (2022). Resveratrol contrasts IL-6 pro-growth effects and promotes autophagy-mediated cancer cell dormancy in 3D ovarian cancer: role of miR-1305 and of its target ARH-I. Cancers., 14(9), 2142. https://doi.org/10.3390/cancers14092142
Amend, S. R., Torga, G., Lin, K., Kostecka, L. G., de Marzo, A., Austin, R. H., & Pienta, K. J. (2019). Polyploid giant cancer cells: unrecognized actuators of tumorigenesis, metastasis, and resistance. The Prostate, 79(13), 1489–1497.
Dudkowska, M., Staniak, K., Bojko, A., & Sikora, E. (2021). Chapter Five - The role of autophagy in escaping therapy-induced polyploidy/senescence. In D. A. Gewirtz, P. B. B. T.-A, & C. R. Fisher (Eds.), Autophagy and senescence in cancer therapy (Vol. 150, pp. 209–247). Academic Press.
Wang, L., Ouyang, M., Xing, S., Zhao, S., Liu, S., Sun, L., & Yu, H. (2022). Mesenchymal stem cells and their derived exosomes promote malignant phenotype of polyploid non-small-cell lung cancer cells through AMPK signaling pathway. Anal. Cell. Pathol, 2022, 8708202. https://doi.org/10.1155/2022/8708202
Peart, T., Ramos Valdes, Y., Correa, R. J. M., Fazio, E., Bertrand, M., McGee, J., et al. (2015). Intact LKB1 activity is required for survival of dormant ovarian cancer spheroids. Oncotarget, 6(26), 22424–22438. https://doi.org/10.18632/oncotarget.4211
Hampsch, R. A., Wells, J. D., Traphagen, N. A., McCleery, C. F., Fields, J. L., Shee, K., et al. (2020). AMPK activation by metformin promotes survival of dormant ER+ breast cancer cells. Clin. Cancer Res., 26(14), 3707–3719. https://doi.org/10.1158/1078-0432.CCR-20-0269
Liu, J., Niu, N., Li, X., Zhang, X., & Sood, A. K. (2022). The life cycle of polyploid giant cancer cells and dormancy in cancer: opportunities for novel therapeutic interventions. Semin. Cancer Biol., 81, 132–144. https://doi.org/10.1016/j.semcancer.2021.10.005
Alhasan, B. A., Gordeev, S. A., Knyazeva, A. R., Aleksandrova, K. V., Margulis, B. A., Guzhova, I. V., & Suvorova, I. I. (2021). The mTOR pathway in pluripotent stem cells: lessons for understanding cancer cell dormancy. Membranes, 11(11). https://doi.org/10.3390/membranes11110858
Bulut-Karslioglu, A., Biechele, S., Jin, H., Macrae, T. A., Hejna, M., Gertsenstein, M., et al. (2016). Inhibition of mTOR induces a paused pluripotent state. Nature, 540(7631), 119–123. https://doi.org/10.1038/nature20578
Hussein, A. M., Wang, Y., Mathieu, J., Margaretha, L., Song, C., Jones, D. C., et al. (2020). Metabolic control over mTOR-dependent diapause-like state. Dev. cell, 52(2), 236–250.e7. https://doi.org/10.1016/j.devcel.2019.12.018
Arun, R. P., Sivanesan, D., Patra, B., Varadaraj, S., & Verma, R. S. (2019). Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Reports, 9(1), 10684. https://doi.org/10.1038/s41598-019-47116-5
Zhang, S., Mercado-Uribe, I., Xing, Z., Sun, B., Kuang, J., & Liu, J. (2014). Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene, 33(1), 116–128.
Díaz-Carballo, D., Saka, S., Klein, J., Rennkamp, T., Acikelli, A. H., Malak, S., et al. (2018). A distinct oncogenerative multinucleated cancer cell serves as a source of stemness and tumor heterogeneity. Cancer Res., 78(9), 2318–2331. https://doi.org/10.1158/0008-5472.CAN-17-1861
Niu, N., Mercado-Uribe, I., & Liu, J. (2017). Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene, 36(34), 4887–4900. https://doi.org/10.1038/onc.2017.72
Scognamiglio, R., Cabezas-Wallscheid, N., Thier, M. C., Altamura, S., Reyes, A., Prendergast, Á. M., et al. (2016). Myc depletion induces a pluripotent dormant state mimicking diapause. Cell, 164(4), 668–680. https://doi.org/10.1016/j.cell.2015.12.033
Dhimolea, E., de Matos Simoes, R., Kansara, D., & Al’Khafaji, A., Bouyssou, J., Weng, X., … Mitsiades, C. S. (2021). An embryonic diapause-like adaptation with suppressed myc activity enables tumor treatment persistence. Cancer Cell, 39(2), 240–256.e11. https://doi.org/10.1016/j.ccell.2020.12.002
Zhao, Y., Wu, H., Xing, X., Ma, Y., Ji, S., Xu, X., et al. (2020). CD13 induces autophagy to promote hepatocellular carcinoma cell chemoresistance through the P38/Hsp27/CREB/ATG7 pathway. J. Pharmacol. Exp. Ther., 374(3), 512–520.
Paillas, S., Causse, A., Marzi, L., De Medina, P., Poirot, M., Denis, V., et al. (2012). MAPK14/p38α confers irinotecan resistance to TP53-defective cells by inducing survival autophagy. Autophagy, 8(7), 1098–1112.
Vera-Ramirez, L., Vodnala, S. K., Nini, R., Hunter, K. W., & Green, J. E. (2018). Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. comm, 9(1), 1944. https://doi.org/10.1038/s41467-018-04070-6
Aqbi, H. F., Tyutyunyk-Massey, L., Keim, R. C., Butler, S. E., Thekkudan, T., Joshi, S., et al. (2018). Autophagy-deficient breast cancer shows early tumor recurrence and escape from dormancy. Oncotarget, 9(31), 22113.
Petherick, K. J., Williams, A. C., Lane, J. D., Ordóñez-Morán, P., Huelsken, J., Collard, T. J., et al. (2013). Autolysosomal β-catenin degradation regulates Wnt-autophagy-p62 crosstalk. The EMBO j., 32(13), 1903–1916.
Lorzadeh, S., Kohan, L., Ghavami, S., & Azarpira, N. (2021). Autophagy and the Wnt signaling pathway: a focus on Wnt/β-catenin signaling. Biochimica et Biophysica Acta (BBA)-Molecular. Cell Res., 1868(3), 118926.
Zada, S., Hwang, J. S., Lai, T. H., Pham, T. M., Ahmed, M., Elashkar, O., et al. (2022). Autophagy-mediated degradation of NOTCH1 intracellular domain controls the epithelial to mesenchymal transition and cancer metastasis. Cell & Biosci., 12(1), 17. https://doi.org/10.1186/s13578-022-00752-3
Ahn, J.-S., Ann, E.-J., Kim, M.-Y., Yoon, J.-H., Lee, H.-J., Jo, E.-H., et al. (2016). Autophagy negatively regulates tumor cell proliferation through phosphorylation dependent degradation of the Notch1 intracellular domain. Oncotarget, 7(48), 79047.
Natsumeda, M., Maitani, K., Liu, Y., Miyahara, H., Kaur, H., Chu, Q., et al. (2016). Targeting notch signaling and autophagy increases cytotoxicity in glioblastoma neurospheres. Brain Pathol., 26(6), 713–723.
Lim, S. M., Mohamad Hanif, E. A., & Chin, S.-F. (2021). Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell & Biosci., 11(1), 56. https://doi.org/10.1186/s13578-021-00570-z
Zhang, B., & Liu, L. (2021). Autophagy is a double-edged sword in the therapy of colorectal cancer. Onco Lett., 21(5), 1–8.
Dower, C. M., Bhat, N., Gebru, M. T., Chen, L., Wills, C. A., Miller, B. A., & Wang, H.-G. (2018). Targeted inhibition of ULK1 promotes apoptosis and suppresses tumor growth and metastasis in neuroblastoma. Mol. Cancer Ther., 17(11), 2365–2376. https://doi.org/10.1158/1535-7163.MCT-18-0176
Hwang, D. Y., Eom, J.-I., Jang, J. E., Jeung, H.-K., Chung, H., Kim, J. S., et al. (2020). ULK1 inhibition as a targeted therapeutic strategy for FLT3-ITD-mutated acute myeloid leukemia. J. Exp. Clin. Cancer Res., 39(1), 85. https://doi.org/10.1186/s13046-020-01580-4
Skah, S., Richartz, N., Duthil, E., Gilljam, K. M., Bindesbøll, C., Naderi, E. H., et al. (2018). cAMP-mediated autophagy inhibits DNA damage-induced death of leukemia cells independent of p53. Oncotarget, 9(54), 30434.
Avsec, D., Jakoš Djordjevič, A. T., Kandušer, M., Podgornik, H., Škerget, M., & Mlinarič-Raščan, I. (2021). Targeting autophagy triggers apoptosis and complements the action of venetoclax in chronic lymphocytic leukemia cells. Cancers. https://doi.org/10.3390/cancers13184557
Deng, J., Thennavan, A., Dolgalev, I., Chen, T., Li, J., Marzio, A., et al. (2021). ULK1 inhibition overcomes compromised antigen presentation and restores antitumor immunity in LKB1-mutant lung cancer. Nat. Cancer, 2(5), 503–514.
Pasquier, B. (2015). SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with MTOR inhibition in tumor cells. Autophagy, 11(4), 725–726. https://doi.org/10.1080/15548627.2015.1033601
Dyczynski, M., Yu, Y., Otrocka, M., Parpal, S., Braga, T., Henley, A. B., et al. (2018). Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 (Vps34) improves the sensitivity of breast cancer cells to Sunitinib. Cancer Lett., 435, 32–43. https://doi.org/10.1016/j.canlet.2018.07.028
Noman, M. Z., Parpal, S., Van Moer, K., Xiao, M., Yu, Y., Arakelian, T., et al. (2022). Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti–PD-1/PD-L1 immunotherapy. Sci. Adv., 6(18), eaax7881. https://doi.org/10.1126/sciadv.aax7881
McAfee, Q., Zhang, Z., Samanta, A., Levi, S. M., Ma, X.-H., Piao, S., et al. (2012). Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc. Natl. Acad. Sci. U.S.A, 109(21), 8253–8258. https://doi.org/10.1073/pnas.1118193109
Cechakova, L., Ondrej, M., Pavlik, V., Jost, P., Cizkova, D., Bezrouk, A., et al. (2019). A potent autophagy inhibitor (Lys05) enhances the impact of ionizing radiation on human lung cancer cells H1299. Int. J. Mol. Sci., 20(23). https://doi.org/10.3390/ijms20235881
Rebecca, V. W., Nicastri, M. C., McLaughlin, N., Fennelly, C., McAfee, Q., Ronghe, A., et al. (2017). A unified approach to targeting the lysosome’s degradative and growth signaling roles. Cancer Disc., 7(11), 1266–1283. https://doi.org/10.1158/2159-8290.CD-17-0741
Rebecca, V. W., Nicastri, M. C., Fennelly, C., Chude, C. I., Barber-Rotenberg, J. S., Ronghe, A., et al. (2019). PPT1 promotes tumor growth and is the molecular target of chloroquine derivatives in cancer. Cancer Disc., 9(2), 220–229. https://doi.org/10.1158/2159-8290.CD-18-0706
Richter, K., Haslbeck, M., & Buchner, J. (2010). The heat shock response: life on the verge of death. Mol. Cell, 40(2), 253–266. https://doi.org/10.1016/j.molcel.2010.10.006
Shevtsov, M., Multhoff, G., Mikhaylova, E., Shibata, A., Guzhova, I., & Margulis, B. (2019). Combination of anti-cancer drugs with molecular chaperone inhibitors. Int. J. Mol. Sci., 20(21), 1–22. https://doi.org/10.3390/ijms20215284
Li, L., Wang, L., You, Q. D., & Xu, X. L. (2020). Heat shock protein 90 inhibitors: an update on achievements, challenges, and future directions. J. Med. Chem., 63(5), 1798–1822. https://doi.org/10.1021/acs.jmedchem.9b00940
Koren, J., & Blagg, B. S. J. (2020). The right tool for the job: an overview of Hsp90 inhibitors. Molecular Cell, 40(2), 253–266.
Xi, C., Hu, Y., Buckhaults, P., Moskophidis, D., & Mivechi, N. F. (2012). Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J. Biol. Chem., 287(42), 35646–35657. https://doi.org/10.1074/jbc.M112.377481
Powell, C. D., Paullin, T. R., Aoisa, C., Menzie, C. J., Ubaldini, A., & Westerheide, S. D. (2016). The heat shock transcription factor HSF1 induces ovarian cancer epithelial-mesenchymal transition in a 3D spheroid growth model. PLoS ONE, 11(12), 1–16. https://doi.org/10.1371/journal.pone.0168389
Scherz-Shouval, R., Santagata, S., Mendillo, M. L., Sholl, L. M., Ben-Aharon, I., Beck, A. H., et al. (2014). The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell, 158(3), 564–578. https://doi.org/10.1016/j.cell.2014.05.045
Mendillo, M. L., Santagata, S., Koeva, M., Bell, G. W., Hu, R., Tamimi, R. M., et al. (2012). HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell, 150(3), 549–562.
Taldone, T., Wang, T., Rodina, A., Pillarsetty, N. V. K., Digwal, C. S., Sharma, S., et al. (2020). A chemical biology approach to the chaperome in cancer—HSP90 and beyond. Cold Spring Harb. Perspect. Biol., 12(4). https://doi.org/10.1101/cshperspect.a034116
Biebl, M. M., & Buchner, J. (2019). Structure, function, and regulation of the hsp90 machinery. Cold Spring Harb. Perspect. Biol., 11(9). https://doi.org/10.1101/cshperspect.a034017
Neckers, L. (2005). Development of small molecule Hsp90 inhibitors: utilizing both forward and reverse chemical genomics for drug identification. Curr. Med. Chem., 10(9), 733–739. https://doi.org/10.2174/0929867033457818
Leskovar, A., Wegele, H., Werbeck, N. D., Buchner, J., & Reinstein, J. (2008). The ATPase cycle of the mitochondrial Hsp90 analog trap1. J. Biol. Chem., 283(17), 11677–11688. https://doi.org/10.1074/jbc.M709516200
Buc Calderon, P., Beck, R., & Glorieux, C. (2019). Targeting hsp90 family members: a strategy to improve cancer cell death. Biochem. Pharmacol., 164(January), 177–187. https://doi.org/10.1016/j.bcp.2019.04.010
Birbo, B., Madu, E. E., Madu, C. O., Jain, A., & Lu, Y. (2021). Role of hsp90 in cancer. Int. J. Mol. Sci., 22(19), 1–19. https://doi.org/10.3390/ijms221910317
Bruschi, M., Petretto, A., Cama, A., Pavanello, M., Bartolucci, M., Morana, G., et al. (2021). Potential biomarkers of childhood brain tumor identified by proteomics of cerebrospinal fluid from extraventricular drainage (EVD). Sci. Reports, 11(1), 1–13. https://doi.org/10.1038/s41598-020-80647-w
Wang, J., Hu, L., Liu, Z., Wang, H., Zhang, H., Song, X., et al. (2022). Identification of heat shock protein 90 as a recurrence related marker in juvenile nasopharyngeal angiofibroma. Am. J. Rhinol., 36(1), 8–17. https://doi.org/10.1177/19458924211012820
Sobhan, P. K., Seervi, M., Joseph, J., Chandrika, B. B., Varghese, S., Santhoshkumar, T. R., & Radhakrishna Pillai, M. (2012). Identification of heat shock protein 90 inhibitors to sensitize drug resistant side population tumor cells using a cell based assay platform. Cancer Lett., 317(1), 78–88. https://doi.org/10.1016/j.canlet.2011.11.009
Liu, B., Shen, Y., Huang, H., Croce, K. D., Wu, M., Fan, Y., et al. (2020). Curcumin derivative C212 inhibits Hsp90 and eliminates both growing and quiescent leukemia cells in deep dormancy. Cell Commun. Signal., 18(1), 1–15.
Schwock, J., Dhani, N., Cao, M. P. J., Zheng, J., Clarkson, R., Radulovich, N., et al. (2009). Targeting focal adhesion kinase with dominant-negative FRNK or Hsp90 inhibitor 17-DMAG suppresses tumor growth and metastasis of SiHa cervical xenografts. Cancer Res., 69(11), 4750–4759. https://doi.org/10.1158/0008-5472.CAN-09-0454
Liu, X., Yan, Z., Huang, L., Guo, M., Zhang, Z., & Guo, C. (2011). Cell surface heat shock protein 90 modulates prostate cancer cell adhesion and invasion through the integrin-β1/ focal adhesion kinase/c-Src signaling pathway. Onco Reports, 25(5), 1343–1351. https://doi.org/10.3892/or.2011.1202
Yoon, S., Yang, H., Ryu, H.-M., Lee, E., Jo, Y., Seo, S., et al. (2022). Integrin αvβ3 induces HSP90 inhibitor resistance via FAK activation in KRAS-mutant non-small cell lung cancer. Cancer Res Treat, 54(3), 767.
Nagaraju, G. P., Mezina, A., Shaib, W. L., Landry, J., & El-Rayes, B. F. (2016). Targeting the Janus-activated kinase-2-STAT3 signalling pathway in pancreatic cancer using the HSP90 inhibitor ganetespib. Eur. J. of Cancer, 52, 109–119. https://doi.org/10.1016/j.ejca.2015.10.057
Cho, T. M., Kim, J. Y., Kim, Y. J., Sung, D., Oh, E., Jang, S., et al. (2019). C-terminal HSP90 inhibitor L80 elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition. Cancer Lett., 447(December 2018), 141–153. https://doi.org/10.1016/j.canlet.2019.01.029
Sun, C., Bai, M., Ke, W., Wang, X., Zhao, X., & Lu, Z. (2021). The HSP90 inhibitor, XL888, enhanced cell apoptosis via downregulating STAT3 after insufficient radiofrequency ablation in hepatocellular carcinoma. Life Sci., 282, 119762. https://doi.org/10.1016/j.lfs.2021.119762
Lee, H. J., Min, H. Y., Yong, Y. S., Ann, J., Nguyen, C. T., La, M. T., et al. (2022). A novel C-terminal heat shock protein 90 inhibitor that overcomes STAT3-Wnt-β-catenin signaling-mediated drug resistance and adverse effects. Theranostics, 27(1), 105–125. https://doi.org/10.7150/thno.63788
Lettini, G., Sisinni, L., Condelli, V., Matassa, D. S., Simeon, V., Maddalena, F., et al. (2016). TRAP1 regulates stemness through Wnt/β-catenin pathway in human colorectal carcinoma. Cell Death Differ, 23(11), 1792–1803. https://doi.org/10.1038/cdd.2016.67
Chen, J. S., Hsu, Y. M., Chen, C. C., Chen, L. L., Lee, C. C., & Huang, T. S. (2010). Secreted heat shock protein 90α induces colorectal cancer cell invasion through CD91/LRP-1 and NF-κB-mediated integrin αV expression. J. Biol. Chem., 285(33), 25458–25466. https://doi.org/10.1074/jbc.M110.139345
de la Mare, J. A., Jurgens, T., & Edkins, A. L. (2017). Extracellular Hsp90 and TGFβ regulate adhesion, migration and anchorage independent growth in a paired colon cancer cell line model. BMC Cancer, 17(1), 1–16. https://doi.org/10.1186/s12885-017-3190-z
Aswad, A., & Liu, T. (2021). Targeting heat shock protein 90 for anti-cancer drug development. In Advances in cancer research (Vol. 152, 1st ed.). Elsevier Inc..
Takamizawa, S., Katsuya, Y., Chen, Y.-N., Mizuno, T., Koyama, T., Sudo, K., et al. (2022). Ocular toxicity of investigational anti-cancer drugs in early phase clinical trials. Invest New Drugs. https://doi.org/10.1007/s10637-022-01321-8
Liew, H. Y., Tan, X. Y., Chan, H. H., Khaw, K. Y., & Ong, Y. S. (2022). Natural HSP90 inhibitors as a potential therapeutic intervention in treating cancers: a comprehensive review. Pharmacol. Res., 181, 106260. https://doi.org/10.1016/j.phrs.2022.106260
Kampinga, H. H., Hageman, J., Vos, M. J., Kubota, H., Tanguay, R. M., Bruford, E. A., et al. (2009). Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones, 14(1), 105–111. https://doi.org/10.1007/s12192-008-0068-7
Balchin, D., Hayer-Hartl, M., & Hartl, F. U. (2016). In vivo aspects of protein folding and quality control. Science, 353(6294). https://doi.org/10.1126/science.aac4354
Rosenzweig, R., Nillegoda, N. B., Mayer, M. P., & Bukau, B. (2019). The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol, 20(11), 665–680. https://doi.org/10.1038/s41580-019-0133-3
Hoogstra-Berends, F., Meijering, R. A. M., Zhang, D., Heeres, A., Loen, L., Seerden, J. P., et al. (2012). Heat shock protein-inducing compounds as therapeutics to restore proteostasis in atrial fibrillation. Trends Cardiovasc. Med., 22(3), 62–68. https://doi.org/10.1016/j.tcm.2012.06.013
Lee, H. J., Shin, S., Kang, J., Han, K. C., Kim, Y. H., Bae, J. W., & Park, K. H. (2020). HSP90 inhibitor, 17-DMAG, alone and in combination with lapatinib attenuates acquired lapatinib-resistance in er-positive, her2-overexpressing breast cancer cell line. Cancers, 12(9), 1–16. https://doi.org/10.3390/cancers12092630
Calderwood, S. K., & Gong, J. (2016). Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem. Sci., 41(4), 311–323. https://doi.org/10.1016/j.tibs.2016.01.003
Boudesco, C., Cause, S., Jego, G., & Garrido, C. (2018). Hsp70: a cancer target inside and outside the cell. Methods mol. biol., 1709, 371–396. https://doi.org/10.1007/978-1-4939-7477-1_27
Margulis, B., Tsimokha, A., Zubova, S., & Guzhova, I. (2020). Molecular chaperones and proteolytic machineries regulate protein homeostasis in aging cells. Cells, 9(5). https://doi.org/10.3390/cells9051308
Ambrose, A. J., & Chapman, E. (2021). Function, therapeutic potential, and inhibition of Hsp70 chaperones. J. Med. Chem., 64(11), 7060–7082.
Rodina, A., Wang, T., Yan, P., Gomes, E. D., Dunphy, M. P. S., Pillarsetty, N., et al. (2016). The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature, 538(7625), 397–401. https://doi.org/10.1038/nature19807
Wang, T., Rodina, A., Dunphy, M. P., Corben, A., Modi, S., Guzman, M. L., et al. (2019). Chaperome heterogeneity and its implications for cancer study and treatment. J. Biol. Chem., 294(6), 2162–2179. https://doi.org/10.1074/jbc.REV118.002811
Kumar, S. J., Stokes, J., Singh, U. P., Scissum Gunn, K., Acharya, A., Manne, U., & Mishra, M. (2016). Targeting Hsp70: a possible therapy for cancer. Cancer Lett, 374(1), 156–166. https://doi.org/10.1016/j.canlet.2016.01.056
Gestwicki, J. E., & Shao, H. (2019). Inhibitors and chemical probes for molecular chaperone networks. J. Biol. Chem., 294(6), 2151–2161. https://doi.org/10.1074/jbc.TM118.002813
Budina-Kolomets, A., Webster, M. R., Leu, J. I. J., Jennis, M., Krepler, C., Guerrini, A., et al. (2016). HSP70 inhibition limits FAK-dependent invasion and enhances the response to melanoma treatment with BRAF inhibitors. Cancer Res., 76(9), 2720–2730. https://doi.org/10.1158/0008-5472.CAN-15-2137
Sun, G., Cao, Y., Xu, Y., De Huai, P. C., Guo, J., Li, M., & Dai, Y. (2019). Overexpression of Hsc70 promotes proliferation, migration, and invasion of human glioma cells. J. Cell. Biochem., 120(6), 10707–10714. https://doi.org/10.1002/jcb.28362
Lin, Y., Peng, N., Zhuang, H., Zhang, D., Wang, Y., & Hua, Z.-C. (2014). Heat shock proteins HSP70 and MRJ cooperatively regulate cell adhesion and migration through urokinase receptor. BMC cancer, 14(1), 1–14.
Ogawa, Y., Nakagami, Y., Ishizaki, R., Yoshida, H., Parkinson, K. M., Robertson, C. N., & Paulson, D. F. (2001). Heat shock protein 70 (HSP70) does not prevent the inhibition of cell growth in DU-145 cells treated with TGF-beta1. Anticancer Res., 21(5), 3341–3347.
Yun, C. H., Yoon, S. Y., Nguyen, T. T., Cho, H. Y., Kim, T. H., Kim, S. T., et al. (2010). Geldanamycin inhibits TGF-β signaling through induction of Hsp70. Arch. Biochem. Biophys., 495(1), 8–13. https://doi.org/10.1016/j.abb.2009.12.003
Shi, F., Ma, M., Zhai, R., Ren, Y., Li, K., Wang, H., et al. (2021). Overexpression of heat shock protein 70 inhibits epithelial-mesenchymal transition and cell migration induced by transforming growth factor-β in A549 cells. Cell Stress Chaperones, 26(3), 505–513. https://doi.org/10.1007/s12192-021-01196-3
Nimmanapalli, R., Gerbino, E., Dalton, W. S., Gandhi, V., & Alsina, M. (2008). HSP70 inhibition reverses cell adhesion mediated and acquired drug resistance in multiple myeloma. Br. J. Haematol., 142(4), 551–561. https://doi.org/10.1111/j.1365-2141.2008.07217.x
Rigg, R. A., Healy, L. D., Nowak, M. S., Mallet, J., Thierheimer, M. L. D., Pang, J., et al. (2016). Heat shock protein 70 regulates platelet integrin activation, granule secretion and aggregation. Am. J. Physiol, 310(7), C568–C575. https://doi.org/10.1152/ajpcell.00362.2015
Xu, N.-W., Chen, Y., Liu, W., Chen, Y.-J., Fan, Z.-M., Liu, M., & Li, L.-J. (2018). Inhibition of JAK2/STAT3 signaling pathway suppresses proliferation of Burkitt’s lymphoma Raji cells via cell cycle progression, apoptosis, and oxidative stress by modulating HSP70. Med. Sci. Monit., 24, 6255–6263. https://doi.org/10.12659/MSM.910170
Matsushima-Nishiwaki, R., Takai, S., Adachi, S., Minamitani, C., Yasuda, E., Noda, T., et al. (2008). Phosphorylated heat shock protein 27 represses growth of hepatocellular carcinoma via inhibition of extracellular signal-regulated kinase. J. Biol. Chem., 283(27), 18852–18860. https://doi.org/10.1074/jbc.M801301200
Chen, S. F., Nieh, S., Jao, S. W., Liu, C. L., Wu, C. H., Chang, Y. C., et al. (2012). Quercetin suppresses drug-resistant spheres via the p38 MAPK-Hsp27 apoptotic pathway in oral cancer cells. PLoS ONE, 7(11). https://doi.org/10.1371/journal.pone.0049275
Willmund, F., Del Alamo, M., Pechmann, S., Chen, T., Albanèse, V., Dammer, E. B., et al. (2013). The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell, 152(1–2), 196–209. https://doi.org/10.1016/j.cell.2012.12.001
Shi, Q. M., Luo, J., Wu, K., Yin, M., Gu, Y. R., & Cheng, X. G. (2016). High level of αB-crystallin contributes to the progression of osteosarcoma. Oncotarget, 7(8), 9007–9016. https://doi.org/10.18632/oncotarget.6928
Volkmann, J., Reuning, U., Rudelius, M., Häfner, N., Schuster, T., Aaron, A. B., et al. (2013). High expression of crystallin αb represents an independent molecular marker for unfavourable ovarian cancer patient outcome and impairs TRAIL- and cisplatin-induced apoptosis in human ovarian cancer cells. Int. J. Cancer., 132(12), 2820–2832. https://doi.org/10.1002/ijc.27975
Yang, M., Li, Y., & Tian, F. (2021). Association between Alpha B-crystallin expression and prognosis in patients with solid tumors: a protocol for systematic review and meta-analysis. Medicine, 100(7), e24831. https://doi.org/10.1097/MD.0000000000024831
Ishida, T., Ishii, Y., Tsuruta, M., Okabayashi, K., Akimoto, S., Koishikawa, K., et al. (2017). Cetuximab promotes SN38 sensitivity via suppression of heat shock protein 27 in colorectal cancer cells with wild-type RAS. Onco Reports, 38(2), 926–932. https://doi.org/10.3892/or.2017.5734
von Rekowski, K. W., König, P., Henze, S., Schlesinger, M., Zawierucha, P., Januchowski, R., & Bendas, G. (2020). Insight into cisplatin-resistance signaling of w1 ovarian cancer cells emerges mtor and hsp27 as targets for sensitization strategies. Int. J. Mol. Sci, 21(23), 1–22. https://doi.org/10.3390/ijms21239240
Sommer, S., Cui, Y., Brewer, G., & Fuqua, S. A. W. (2005). The c-Yes 3′-UTR contains adenine/uridine-rich elements that bind AUF1 and HuR involved in mRNA decay in breast cancer cells. J. Steroid Biochem. Mol. Biol., 97(3), 219–229. https://doi.org/10.1016/j.jsbmb.2005.09.002
Nikotina, A. D., Koludarova, L., Komarova, E. Y., Mikhaylova, E. R., Aksenov, N. D., Suezov, R., et al. (2018). Discovery and optimization of cardenolides inhibiting HSF1 activation in human colon HCT-116 cancer cells. Oncotarget, 9(43), 27268.
Guan, L., Zou, Q., Liu, Q., Lin, Y., & Chen, S. (2020). HSP90 inhibitor ganetespib (STA-9090) inhibits tumor growth in c-Myc-dependent esophageal squamous cell carcinoma. OncoTargets Ther., 13, 2997–3011. https://doi.org/10.2147/OTT.S245813
Joshi, S., Gomes, E. D. G., Wang, T., Corben, A., Taldone, T., Gandu, S., et al. (2021). Pharmacologically controlling protein-protein interactions through epichaperomes for therapeutic vulnerability in cancer. Commun. Biol., 4(1), 1–20. https://doi.org/10.1038/s42003-021-02842-3
Hong, S. K., Starenki, D., Johnson, O. T., Gestwicki, J. E., & Park, J. I. (2022). Analogs of the heat shock protein 70 inhibitor MKT-077 suppress medullary thyroid carcinoma cells. Int. J. Mol. Sci., 23(3). https://doi.org/10.3390/ijms23031063
Sojka, D. R., Hasterok, S., Vydra, N., Toma-jonik, A., Wieczorek, A., Gogler-pigłowska, A., & Scieglinska, D. (2021). Inhibition of the heat shock protein a (Hspa) family potentiates the anticancer effects of manumycin A. Cells, 10(6), 1–15. https://doi.org/10.3390/cells10061418
Hyun, S. Y., Le, H. T., Min, H.-Y., Pei, H., Lim, Y., Song, I., et al. (2021). Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70. Theranostics, 11(6), 2932–2952. https://doi.org/10.7150/thno.49876
Chen, R., Qiao, Y., Hu, W., Cheng, Q., Xie, H., Zhou, L., et al. (2019). LY2228820 induces synergistic anti-cancer effects with anti-microtubule chemotherapeutic agents independent of P-glycoprotein in multidrug resistant cancer cells. Am. J. Cancer Res., 9(10), 2216.
Almanza, A., Carlesso, A., Chintha, C., Creedican, S., Doultsinos, D., Leuzzi, B., et al. (2019). Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. The FEBS j., 286(2), 241–278.
Hetz, C., & Papa, F. R. (2018). The unfolded protein response and cell fate control. Mol Cell, 69(2), 169–181. https://doi.org/10.1016/j.molcel.2017.06.017
Robinson, C. M., Talty, A., Logue, S. E., Mnich, K., Gorman, A. M., & Samali, A. (2021). An emerging role for the unfolded protein response in pancreatic cancer. Cancers, 13(2). https://doi.org/10.3390/cancers13020261
Huang, J., Pan, H., Wang, J., Wang, T., Huo, X., Ma, Y., et al. (2021). Unfolded protein response in colorectal cancer. Cell & Bio., 11(1), 26. https://doi.org/10.1186/s13578-021-00538-z
Hart, L. S., Cunningham, J. T., Datta, T., Dey, S., Tameire, F., Lehman, S. L., et al. (2012). ER stress–mediated autophagy promotes Myc-dependent transformation and tumor growth. J. Clin. Investig., 122(12), 4621–4634.
Corazzari, M., Rapino, F., Ciccosanti, F., Giglio, P., Antonioli, M., Conti, B., et al. (2015). Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ, 22(6), 946–958.
Madden, E., Logue, S. E., Healy, S. J., Manie, S., & Samali, A. (2019). The role of the unfolded protein response in cancer progression: from oncogenesis to chemoresistance. Bio. of the Cell, 111(1), 1–17. https://doi.org/10.1111/boc.201800050
Chen, J., Lynn, E. G., Yousof, T. R., Sharma, H., MacDonald, M. E., Byun, J. H., et al. (2022). Scratching the surface—an overview of the roles of cell surface GRP78 in cancer. Biomedicines, 10(5), 1098. https://doi.org/10.3390/biomedicines10051098
Tosh, D. K., Brackett, C. M., Jung, Y.-H., Gao, Z.-G., Banerjee, M., Blagg, B. S. J., & Jacobson, K. A. (2021). Biological evaluation of 5′-(N-Ethylcarboxamido) adenosine analogues as Grp94-selective inhibitors. ACS Med. Chem. Lett., 12(3), 373–379.
Xu, X., Chiu, J., Chen, S., & Fang, C. (2021). Pathophysiological roles of cell surface and extracellular protein disulfide isomerase and their molecular mechanisms. Br. J. Pharmacol., 178(15), 2911–2930. https://doi.org/10.1111/bph.15493
Hsu, S.-K., Chiu, C.-C., Dahms, H.-U., Chou, C.-K., Cheng, C.-M., Chang, W.-T., et al. (2019). Unfolded protein response (UPR) in survival, dormancy, immunosuppression, metastasis, and treatments of cancer cells. Int. J. Mol. Sci., 20(10). https://doi.org/10.3390/ijms20102518
Payne, K. K. (2022). Cellular stress responses and metabolic reprogramming in cancer progression and dormancy. Semin. Cancer Biol., 78, 45–48. https://doi.org/10.1016/j.semcancer.2021.06.004
Ranganathan, A. C., Zhang, L., Adam, A. P., & Aguirre-Ghiso, J. A. (2006). Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase–like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer res., 66(3), 1702–1711.
Bartkowiak, K., Kwiatkowski, M., Buck, F., Gorges, T. M., Nilse, L., Assmann, V., et al. (2015). Disseminated tumor cells persist in the bone marrow of breast cancer patients through sustained activation of the unfolded protein response. Cancer res., 75(24), 5367–5377.
Brewer, J. W., & Diehl, J. A. (2000). PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. U.S.A., 97(23), 12625–12630.
Ranganathan, A. C., Ojha, S., Kourtidis, A., Conklin, D. S., & Aguirre-Ghiso, J. A. (2008). Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer res., 68(9), 3260–3268.
Harding, H. P., Zhang, Y., Bertolotti, A., Zeng, H., & Ron, D. (2000). Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. cell, 5(5), 897–904.
Cullinan, S. B., & Diehl, J. A. (2004). PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem., 279(19), 20108–20117.
Sandoval, M. V., Fluegen, G., Staschke, K. A., Calvo-Vidal, V., & Aguirre-Ghiso, J. A. (2016). Abstract A45: PERK-inhibition as a possible therapy for hypoxia-induced solitary dormant tumor cells. Cancer Res., 76(7_Supplement), A45–A45.
Schewe, D. M., & Aguirre-Ghiso, J. A. (2008). ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc. Natl. Acad. Sci. U.S.A., 105(30), 10519–10524.
Back, S. H., Lee, K., Vink, E., & Kaufman, R. J. (2006). Cytoplasmic IRE1α-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J. Biol. Chem., 281(27), 18691–18706.
Romero-Ramirez, L., Cao, H., Nelson, D., Hammond, E., Lee, A.-H., Yoshida, H., et al. (2004). XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer res., 64(17), 5943–5947.
Chen, X., Iliopoulos, D., Zhang, Q., Tang, Q., Greenblatt, M. B., Hatziapostolou, M., et al. (2014). XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature, 508(7494), 103–107. https://doi.org/10.1038/nature13119
Hu, F., Han, J., Zhai, B., Ming, X., Zhuang, L., Liu, Y., et al. (2014). Blocking autophagy enhances the apoptosis effect of bufalin on human hepatocellular carcinoma cells through endoplasmic reticulum stress and JNK activation. Apoptosis, 19(1), 210–223. https://doi.org/10.1007/s10495-013-0914-7
Rouschop, K. M. A., van den Beucken, T., Dubois, L., Niessen, H., Bussink, J., Savelkouls, K., et al. (2010). The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investig., 120(1), 127–141.
B’chir, W., Maurin, A.-C., Carraro, V., Averous, J., Jousse, C., Muranishi, Y., et al. (2013). The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res., 41(16), 7683–7699.
Li, Y., Cook, K. L., Yu, W., Jin, L., Bouker, K. B., Clarke, R., & Hilakivi-Clarke, L. (2021). Inhibition of antiestrogen-promoted pro-survival autophagy and tamoxifen resistance in breast cancer through vitamin D receptor. Nutrients, 13(5). https://doi.org/10.3390/nu13051715
Cook, K. L., Clarke, P. A. G., Parmar, J., Hu, R., Schwartz-Roberts, J. L., Abu-Asab, M., et al. (2014). Knockdown of estrogen receptor-α induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death. The FASEB J., 28(9), 3891–3905. https://doi.org/10.1096/fj.13-247353
Rong, H., Anni, W., Lu, J., Alan, Z., & B., R. R., Hong-Bin, F., & Robert, C. (2015). NF-κB signaling is required for XBP1 (unspliced and spliced)-mediated effects on antiestrogen responsiveness and cell fate decisions in breast cancer. Mol. Cell. Biol., 35(2), 379–390. https://doi.org/10.1128/MCB.00847-14
Salaroglio, I. C., Panada, E., Moiso, E., Buondonno, I., Provero, P., Rubinstein, M., et al. (2017). PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol Cancer, 16(1), 91. https://doi.org/10.1186/s12943-017-0657-0
Ni, M., Zhang, Y., & Lee, A. S. (2011). Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Bio. J, 434(2), 181–188. https://doi.org/10.1042/BJ20101569
Peng, Y., Li, Z., & Li, Z. (2013). GRP78 secreted by tumor cells stimulates differentiation of bone marrow mesenchymal stem cells to cancer-associated fibroblasts. Biochem. Biophys. Res. Commun, 440(4), 558–563.
Hernandez, I., & Cohen, M. (2022). Linking cell-surface GRP78 to cancer: from basic research to clinical value of GRP78 antibodies. Cancer Lett., 524, 1–14. https://doi.org/10.1016/j.canlet.2021.10.004
Gopal, U., Mowery, Y., Young, K., & Pizzo, S. V. (2019). Targeting cell surface GRP78 enhances pancreatic cancer radiosensitivity through YAP/TAZ protein signaling. J. Biol. Chem., 294(38), 13939–13952.
Chang, H.-L., Chen, H.-A., Bamodu, O. A., Lee, K.-F., Tzeng, Y.-M., Lee, W.-H., & Tsai, J.-T. (2018). Ovatodiolide suppresses yes-associated protein 1-modulated cancer stem cell phenotypes in highly malignant hepatocellular carcinoma and sensitizes cancer cells to chemotherapy in vitro. Tox. in Vitro, 51, 74–82. https://doi.org/10.1016/j.tiv.2018.04.010
Zhang, L., Li, Z., Fan, Y., Li, H., Li, Z., & Li, Y. (2015). Overexpressed GRP78 affects EMT and cell-matrix adhesion via autocrine TGF-β/Smad2/3 signaling. Int. J. Biochem. Cell Biol., 64, 202–211. https://doi.org/10.1016/j.biocel.2015.04.012
Nami, B., Ghasemi-Dizgah, A., & Vaseghi, A. (2016). Overexpression of molecular chaperons GRP78 and GRP94 in CD44hi/CD24lo breast cancer stem cells. BioImpacts: BI, 6(2), 105.
Bartkowiak, K., Effenberger, K. E., Harder, S., Andreas, A., Buck, F., Peter-Katalinic, J., et al. (2010). Discovery of a novel unfolded protein response phenotype of cancer stem/progenitor cells from the bone marrow of breast cancer patients. J. Proteome Res., 9(6), 3158–3168.
Sanz-Pamplona, R., Aragüés, R., Driouch, K., Martín, B., Oliva, B., Gil, M., et al. (2011). Expression of endoplasmic reticulum stress proteins is a candidate marker of brain metastasis in both ErbB-2+ and ErbB-2− primary breast tumors. Am. J. Clin. Pathol., 179(2), 564–579.
Martínez-Aranda, A., Hernández, V., Guney, E., Muixí, L., Foj, R., Baixeras, N., et al. (2015). FN14 and GRP94 expression are prognostic/predictive biomarkers of brain metastasis outcome that open up new therapeutic strategies. Oncotarget, 6(42), 44254.
Zhang, L., Wang, S., Wangtao, W. Y., Wang, J., Jiang, L., Li, S., Hu, X., & Wang, Q. (2009). Upregulation of GRP78 and GRP94 and its function in chemotherapy resistance to VP-16 in human lung cancer cell line SK-MES-1. Cancer inv., 27(4), 453–458.
Santana-Codina, N., Muixí, L., Foj, R., Sanz-Pamplona, R., Badia-Villanueva, M., Abramowicz, A., et al. (2020). GRP94 promotes brain metastasis by engaging pro-survival autophagy. Neuro-onco, 22(5), 652–664.
Misra, U. K., Payne, S., & Pizzo, S. V. (2011). Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: a role for secreted prostate-specific antigen. J. Biol. Chem., 286(2), 1248–1259.
Misra, U. K., Payne, S., & Pizzo, S. V. (2013). The monomeric receptor binding domain of tetrameric α2-macroglobulin binds to cell surface GRP78 triggering equivalent activation of signaling cascades. Biochemistry, 52(23), 4014–4025. https://doi.org/10.1021/bi400376s
Liu, R., Li, X., Gao, W., Zhou, Y., Wey, S., Mitra, S. K., et al. (2013). Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin. Cancer Res., 19(24), 6802–6811. https://doi.org/10.1158/1078-0432.CCR-13-1106
Lin, Y. G., Shen, J., Yoo, E., Liu, R., Yen, H.-Y., Mehta, A., et al. (2015). Targeting the glucose-regulated protein-78 abrogates Pten-null driven AKT activation and endometrioid tumorigenesis. Oncogene, 34(43), 5418–5426.
Wey, S., Luo, B., Tseng, C.-C., Ni, M., Zhou, H., Fu, Y., et al. (2012). Inducible knockout of GRP78/BiP in the hematopoietic system suppresses Pten-null leukemogenesis and AKT oncogenic signaling. Am. J. Hematol., 119(3), 817–825.
Kelber, J. A., Panopoulos, A. D., Shani, G., Booker, E. C., Belmonte, J. C., Vale, W. W., & Gray, P. C. (2009). Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene, 28(24), 2324–2336. https://doi.org/10.1038/onc.2009.97
Francescangeli, F., Contavalli, P., De Angelis, M. L., Baiocchi, M., Gambara, G., Pagliuca, A., et al. (2015). Dynamic regulation of the cancer stem cell compartment by Cripto-1 in colorectal cancer. Cell Death Differ., 22(10), 1700–1713. https://doi.org/10.1038/cdd.2015.19
Karkampouna, S., van der Helm, D., Gray, P. C., Chen, L., Klima, I., Grosjean, J., et al. (2018). CRIPTO promotes an aggressive tumour phenotype and resistance to treatment in hepatocellular carcinoma. The J of Patho., 245(3), 297–310. https://doi.org/10.1002/path.5083
Francescangeli, F., De Angelis, M. L., Rossi, R., Sette, G., Eramo, A., Boe, A., et al. (2022). CRIPTO is a marker of chemotherapy-induced stem cell expansion in non-small cell lung cancer. Front. in Onco, 12. https://doi.org/10.3389/fonc.2022.830873
Ishii, H., Afify, S. M., Hassan, G., Salomon, D. S., & Seno, M. (2021). Cripto-1 as a potential target of cancer stem cells for immunotherapy. Cancers. 13, 2491. https://doi.org/10.3390/cancers13102491
Zoni, E., Chen, L., Karkampouna, S., Granchi, Z., Verhoef, E. I., La Manna, F., et al. (2017). CRIPTO and its signaling partner GRP78 drive the metastatic phenotype in human osteotropic prostate cancer. Oncogene, 36(33), 4739–4749. https://doi.org/10.1038/onc.2017.87
Yun, S., Yun, C. W., Lee, J. H., Kim, S., & Lee, S. H. (2018). Cripto enhances proliferation and survival of mesenchymal stem cells by up-regulating JAK2/STAT3 pathway in a GRP78-dependent manner. Biomol. Ther., 26(5), 464–473. https://doi.org/10.4062/biomolther.2017.099
Zeng, Q., Gao, Y., & Zhou, Y. (2022). Understanding the role of Cripto-1 in cancer progression and therapeutic strategies. Clin Transl Oncol. https://doi.org/10.1007/s12094-022-03023-2
Du, T., Li, H., Fan, Y., Yuan, L., Guo, X., Zhu, Q., et al. (2019). The deubiquitylase OTUD3 stabilizes GRP78 and promotes lung tumorigenesis. Nat. Comm., 10(1), 2914. https://doi.org/10.1038/s41467-019-10824-7
Rangel, D. F., Dubeau, L., Park, R., Chan, P., Ha, D. P., Pulido, M. A., et al. (2021). Endoplasmic reticulum chaperone GRP78/BiP is critical for mutant Kras-driven lung tumorigenesis. Oncogene, 40(20), 3624–3632. https://doi.org/10.1038/s41388-021-01791-9
Li, Z., Zhang, L., Zhao, Y., Li, H., Xiao, H., Fu, R., et al. (2013). Cell-surface GRP78 facilitates colorectal cancer cell migration and invasion. Int. J. Biochem. Cell Biol., 45(5), 987–994. https://doi.org/10.1016/j.biocel.2013.02.002
Yuan, X. P., Dong, M., Li, X., & Zhou, J. P. (2015). GRP78 promotes the invasion of pancreatic cancer cells by FAK and JNK. Mol. Cell. Biochem., 398(1), 55–62. https://doi.org/10.1007/s11010-014-2204-2
Zhao, S., Li, H., Wang, Q., Su, C., Wang, G., Song, H., et al. (2015). The role of c-Src in the invasion and metastasis of hepatocellular carcinoma cells induced by association of cell surface GRP78 with activated α2M. BMC Cancer, 15(1), 389. https://doi.org/10.1186/s12885-015-1401-z
Liu, Y., Ji, W., Yue, N., & Zhou, W. (2021). Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway. Open Life Sci., 1082, 16(1), –1090. https://doi.org/10.1515/biol-2021-0108
Zhong, Y., & Lan, J. (2022). Overexpression of Eukaryotic translation initiation factor 3D induces stem cell-like properties and metastasis in cervix cancer by activating FAK through inhibiting degradation of GRP78. Bioengineered, 13(1), 1952–1961. https://doi.org/10.1080/21655979.2021.2024336
Yao, X., Liu, H., Zhang, X., Zhang, L., Li, X., Wang, C., & Sun, S. (2015). Cell surface GRP78 accelerated breast cancer cell proliferation and migration by activating STAT3. PloS one, 10(5), e0125634.
Niu, Z., Wang, M., Zhou, L., Yao, L., Liao, Q., & Zhao, Y. (2015). Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci Reports, 5(1), 16067. https://doi.org/10.1038/srep16067
Tseng, C.-C., Stanciauskas, R., Zhang, P., Woo, D., Wu, K., Kelly, K., et al. (2019). GRP78 regulates CD44v membrane homeostasis and cell spreading in tamoxifen-resistant breast cancer. Life sci alliance, 2(4).
Tseng, C.-C., Zhang, P., & Lee, A. S. (2019). The COOH-terminal proline-rich region of GRP78 is a key regulator of its cell surface expression and viability of tamoxifen-resistant breast cancer cells. Neoplasia, 21(8), 837–848. https://doi.org/10.1016/j.neo.2019.05.008
Chiu, C.-C., Lee, L.-Y., Li, Y.-C., Chen, Y.-J., Lu, Y.-C., Li, Y.-L., et al. (2013). Grp78 as a therapeutic target for refractory head–neck cancer with CD24−CD44+ stemness phenotype. Cancer Gene Ther., 20(11), 606–615. https://doi.org/10.1038/cgt.2013.64
Dauer, P., Sharma, N. S., Gupta, V. K., Durden, B., Hadad, R., Banerjee, S., et al. (2019). ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness.”. Cell Death Dis., 10(2), 132. https://doi.org/10.1038/s41419-019-1408-5
Schneider, M., Winkler, K., Kell, R., Pfaffl, M. W., Atkinson, M. J., & Moertl, S. (2022). The chaperone protein GRP78 promotes survival and migration of head and neck cancer after direct radiation exposure and extracellular vesicle-transfer. Front Oncol, 12.
Li, B., Cheng Liang, X., Yang Peng, Y., & Li Quan, Z. (2013). GRP78 mediates radiation resistance of a stem cell-like subpopulation within the MCF-7 breast cancer cell line. Oncol Rep, 30(5), 2119–2126. https://doi.org/10.3892/or.2013.2710
Conner, C., Lager, T. W., Guldner, I. H., Wu, M.-Z., Hishida, Y., Hishida, T., et al. (2020). Cell surface GRP78 promotes stemness in normal and neoplastic cells. Sci. reports, 10(1), 1–11.
Mo, L., Bachelder, R. E., Kennedy, M., Chen, P.-H., Chi, J.-T., Berchuck, A., et al. (2015). Syngeneic murine ovarian cancer model reveals that ascites enriches for ovarian cancer stem-like cells expressing membrane GRP78. Mol. Cancer Ther., 14(3), 747–756. https://doi.org/10.1158/1535-7163.MCT-14-0579
Lager, T. W., Conner, C., Keating, C. R., Warshaw, J. N., & Panopoulos, A. D. (2021). Cell surface GRP78 and Dermcidin cooperate to regulate breast cancer cell migration through Wnt signaling. Oncogene, 40(23), 4050–4059. https://doi.org/10.1038/s41388-021-01821-6
Xiong, H., Xiao, H., Luo, C., Chen, L., Liu, X., Hu, Z., et al. (2019). GRP78 activates the Wnt/HOXB9 pathway to promote invasion and metastasis of hepatocellular carcinoma by chaperoning LRP6. Exp. Cell Res., 383(1), 111493.
Liu, B., Staron, M., Hong, F., Wu, B. X., Sun, S., Morales, C., et al. (2013). Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. U.S.A., 110(17), 6877–6882. https://doi.org/10.1073/pnas.1302933110
Hua, Y., White-Gilbertson, S., Kellner, J., Rachidi, S., Usmani, S. Z., Chiosis, G., et al. (2013). Molecular chaperone gp96 is a novel therapeutic target of multiple myelomaRoles of gp96 in regulating myeloma. Clin. Cancer Res., 19(22), 6242–6251.
Hu, T., Xie, N., Qin, C., Wang, J., & You, Y. (2015). Glucose-regulated protein 94 is a novel glioma biomarker and promotes the aggressiveness of glioma via Wnt/β-catenin signaling pathway. Tumor Biol., 36(12), 9357–9364.
Shen, J., Yao, L., Lin, Y. G., DeMayo, F. J., Lydon, J. P., Dubeau, L., & Lee, A. S. (2016). Glucose-regulated protein 94 deficiency induces squamous cell metaplasia and suppresses PTEN-null driven endometrial epithelial tumor development. Oncotarget, 7(12), 14885.
Hou, J., Li, X., Li, C., Sun, L., Zhao, Y., Zhao, J., & Meng, S. (2015). Plasma membrane gp96 enhances invasion and metastatic potential of liver cancer via regulation of uPAR. Mol. Oncol., 9(7), 1312–1323. https://doi.org/10.1016/j.molonc.2015.03.004
Duan, X., Iwanowycz, S., Ngoi, S., Hill, M., Zhao, Q., & Liu, B. (2021). Molecular chaperone GRP94/GP96 in cancers: oncogenesis and therapeutic target. Front Oncol, 11, 629846.
Backer, J. M., Krivoshein, A. V., Hamby, C. V., Pizzonia, J., Gilbert, K. S., Ray, Y. S., et al. (2009). Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells. Neoplasia, 11(11), 1165–IN11.
Rasche, L., Duell, J., Morgner, C., Chatterjee, M., Hensel, F., Rosenwald, A., et al. (2013). The natural human IgM antibody PAT-SM6 induces apoptosis in primary human multiple myeloma cells by targeting heat shock protein GRP78. PloS one, 8(5), e63414.
Axten, J. M., Medina, J. R., Feng, Y., Shu, A., Romeril, S. P., Grant, S. W., et al. (2012). Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem., 55(16), 7193–7207. https://doi.org/10.1021/jm300713s
Atkins, C., Liu, Q., Minthorn, E., Zhang, S.-Y., Figueroa, D. J., Moss, K., et al. (2013). Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res., 73(6), 1993–2002. https://doi.org/10.1158/0008-5472.CAN-12-3109
Shi, Z., Yu, X., Yuan, M., Lv, W., Feng, T., Bai, R., & Zhong, H. (2019). Activation of the PERK-ATF4 pathway promotes chemo-resistance in colon cancer cells. Sci. reports, 9(1), 1–8.
Sheng, X., Nenseth, H. Z., Qu, S., Kuzu, O. F., Frahnow, T., Simon, L., et al. (2019). IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling. Nat. Comm., 10(1), 323. https://doi.org/10.1038/s41467-018-08152-3
Papandreou, I., Denko, N. C., Olson, M., Van Melckebeke, H., Lust, S., Tam, A., et al. (2011). Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood, 117(4), 1311–1314. https://doi.org/10.1182/blood-2010-08-303099
Ming, J., Ruan, S., Wang, M., Ye, D., Fan, N., Meng, Q., et al. (2015). A novel chemical, STF-083010, reverses tamoxifen-related drug resistance in breast cancer by inhibiting IRE1/XBP1. Oncotarget, 6(38).
Cho, J., Min, H.-Y., Pei, H., Wei, X., Sim, J. Y., Park, S.-H., et al. (2020). The ATF6-EGF pathway mediates the awakening of slow-cycling chemoresistant cells and tumor recurrence by stimulating tumor angiogenesis. Cancers, 12(7), 1772. https://doi.org/10.3390/cancers12071772
Gallagher, C. M., Garri, C., Cain, E. L., Ang, K. K.-H., Wilson, C. G., Chen, S., et al. (2016). Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. elife, 5, e11878.
Tang, F., Hu, P., Yang, Z., Xue, C., Gong, J., Sun, S., et al. (2017). SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways. Oncol Rep, 37(6), 3449–3458. https://doi.org/10.3892/or.2017.5635
Qiu, L., Zhou, G., & Cao, S. (2020). Targeted inhibition of ULK1 enhances daunorubicin sensitivity in acute myeloid leukemia. Life Sci, 243, 117234. https://doi.org/10.1016/j.lfs.2019.117234
Noman, M. Z., Parpal, S., Van Moer, K., Xiao, M., Yu, Y., Arakelian, T., et al. (2020). Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti–PD-1/PD-L1 immunotherapy. Sci. adv., 6(18), eaax7881.
Lee, Y. S., Bradley, S. T., Gurel, Z., Nickel, K. P., & Kimple, R. J. (2021). Mitophagy induction by ROS-PINK1 signaling protects head and neck cancer from radiotherapy. Int. J. Radiat. Oncol. Biol. Phys., 111(3), S56. https://doi.org/10.1016/j.ijrobp.2021.07.145
Marastoni, S., Madariaga, A., Pesic, A., Nair, S. N., Li, Z. J., Shalev, Z., et al. (2022). Repurposing itraconazole and hydroxychloroquine to target lysosomal homeostasis in epithelial ovarian cancer. Cancer Treat Res Commun, 2(5), 293–306. https://doi.org/10.1158/2767-9764.CRC-22-0037
Anand, K., Niravath, P., Patel, T., Ensor, J., Rodriguez, A., Boone, T., et al. (2021). A phase II study of the efficacy and safety of chloroquine in combination with taxanes in the treatment of patients with advanced or metastatic anthracycline-refractory breast cancer. Clin. Breast Cancer, 21(3), 199–204. https://doi.org/10.1016/j.clbc.2020.09.015
Ma, X.-H., Piao, S.-F., Dey, S., Mcafee, Q., Karakousis, G., Villanueva, J., et al. (2014). Targeting ER stress–induced autophagy overcomes BRAF inhibitor resistance in melanoma. J. Clin. Investig., 124(3), 1406–1417.
DeVorkin, L., Hattersley, M., Kim, P., Ries, J., Spowart, J., Anglesio, M. S., et al. (2017). Autophagy inhibition enhances sunitinib efficacy in clear cell ovarian carcinoma. Mol. Cancer Res, 15(3), 250–258. https://doi.org/10.1158/1541-7786.MCR-16-0132
Jhaveri, K., Wang, R., Teplinsky, E., Chandarlapaty, S., Solit, D., Cadoo, K., et al. (2017). A phase I trial of ganetespib in combination with paclitaxel and trastuzumab in patients with human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer. Breast Cancer Res., 19(1), 1–8. https://doi.org/10.1186/s13058-017-0879-5
Parris, J. L. D., Barnoud, T., Leu, J. I.-J., Leung, J. C., Ma, W., Kirven, N. A., et al. (2021). HSP70 inhibition blocks adaptive resistance and synergizes with MEK inhibition for the treatment of NRAS-mutant melanoma. Cancer Treat Res Commun, 1(1), 17–29.
Nappi, L., Aguda, A. H., Al Nakouzi, N., Lelj-Garolla, B., Beraldi, E., Lallous, N., et al. (2020). Ivermectin inhibits HSP27 and potentiates efficacy of oncogene targeting in tumor models. J. Clin. Investig., 130(2), 699–714. https://doi.org/10.1172/JCI130819
Alasiri, G., Jiramongkol, Y., Trakansuebkul, S., Ke, H.-L., Mahmud, Z., Intuyod, K., & Lam, E. W.-F. (2020). Reciprocal regulation between GCN2 (eIF2AK4) and PERK (eIF2AK3) through the JNK-FOXO3 axis to modulate cancer drug resistance and clonal survival. Mol. Cell. Endocrinol., 515, 110932. https://doi.org/10.1016/j.mce.2020.110932
Lin, J.-C., Yang, P.-M., & Liu, T.-P. (2021). PERK/ATF4-dependent ZFAS1 upregulation is associated with sorafenib resistance in hepatocellular carcinoma cells. Int. J. Mol. Sci., 22(11), 5848.
Logue, S. E., McGrath, E. P., Cleary, P., Greene, S., Mnich, K., Almanza, A., et al. (2018). Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Comm., 9(1), 3267. https://doi.org/10.1038/s41467-018-05763-8
Creedican, S., Robinson, C. M., Mnich, K., Islam, M. N., Szegezdi, E., Clifford, R., et al. (2022). Inhibition of IRE1α RNase activity sensitizes patient-derived acute myeloid leukaemia cells to proteasome inhibitors. J Cell Mol Med, 26(16), 4629–4633. https://doi.org/10.1111/jcmm.17479
Huang, Y., Yuan, K., Tang, M., Yue, J., Bao, L., Wu, S., et al. (2021). Melatonin inhibiting the survival of human gastric cancer cells under ER stress involving autophagy and Ras-Raf-MAPK signalling. J Cell Mol Med, 25(3), 1480–1492.
Zhao, R., Lv, Y., Feng, T., Zhang, R., Ge, L., Pan, J., et al. (2022). ATF6α promotes prostate cancer progression by enhancing PLA2G4A-mediated arachidonic acid metabolism and protecting tumor cells against ferroptosis. The Prostate, 82(5), 617–629. https://doi.org/10.1002/pros.24308
Abbreviations
ATGs, autophagy-related genes; BM, bone marrow; CQ, сhloroquine; CRC, colorectal cancer cells; CSCs, cancer stem cells; CTCs, circulating tumor cells; DAMPs, damage-associated molecular patterns; DTCs, disseminated tumor cells; DTPs, drug-tolerant persisters; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; ER, endoplasmic reticulum; ERAD, ER-associated degradation; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GRP94, glucose-regulated protein 94 kDa; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; HCQ, hydroxychloroquine; HNSCC, head and neck squamous cell carcinoma; HIF1α, hypoxia-inducible factor 1α; HMGB1, high-mobility group box 1; HSP, heat shock protein; LKB1, liver kinase B1; MAPK, mitogen-activated protein kinase; MICs, metastasis-initiating cells; MLCK, myosin light chain kinase; MSCs, mesenchymal stem cells; NICD, NOTCH intracellular domain; NR2F1, Orphan nuclear receptor 1; NSCLC, non-small cell lung cancer; PDAC, pancreatic ductal adenocarcinoma; PDI, protein disulfide isomerase; PDX, patient-derived xenografts; PGCCs, polyploid giant cancer cells; PSA, prostate-specific antigen; ROS, reactive oxygen species; SPARC, secreted protein acidic and rich in cysteine; TBK1, Tank binding kinase 1; TEAD, transcriptional enhanced associated domain; TICs, tumor-initiating cells; TME, tumor microenvironment; TNBC, triple-negative breast cancer; TRAP1, Tumor necrosis factor receptor-associated protein 1; UPR, unfolded protein response; YAP, YES-associated protein; ZEB2, zinc finger E-box binding homeobox 2; 3-MA, 3-methyladenine
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation (Grant No. 075-15-2020-773).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alhasan, B., Mikeladze, M., Guzhova, I. et al. Autophagy, molecular chaperones, and unfolded protein response as promoters of tumor recurrence. Cancer Metastasis Rev 42, 217–254 (2023). https://doi.org/10.1007/s10555-023-10085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-023-10085-3