Abstract
Obesity represents an important risk factor for prostate cancer, driving more aggressive disease, chemoresistance, and increased mortality. White adipose tissue (WAT) overgrowth in obesity is central to the mechanisms that lead to these clinical observations. Adipose stromal cells (ASCs), the progenitors to mature adipocytes and other cell types in WAT, play a vital role in driving PCa aggressiveness. ASCs produce numerous factors, especially chemokines, including the chemokine CXCL12, which is involved in driving EMT and chemoresistance in PCa. A greater understanding of the impact of WAT in obesity-induced progression of PCa and the underlying mechanisms has begun to provide opportunities for developing interventional strategies for preventing or offsetting these critical events. These include weight loss regimens, therapeutic targeting of ASCs, use of calorie restriction mimetic compounds, and combinations of compounds as well as specific receptor targeting strategies.

Similar content being viewed by others
References
Hales, C. M., Carroll, M. D., Fryar, C. D., & Ogden, C. L. (2020). Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, (360), 1–8.
Nomura, A. M. (2001). Body size and prostate cancer. Epidemiologic Reviews, 23(1), 126–131.
Porter, M. P., & Stanford, J. L. (2005). Obesity and the risk of prostate cancer. Prostate, 62(4), 316–321.
Roehl, K. A., Han, M., Ramos, C. G., Antenor, J. A., & Catalona, W. J. (2004). Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: Long-term results. Journal of Urology, 172(3), 910–914. https://doi.org/10.1097/01.ju.0000134888.22332.bb
Pound, C. R., Partin, A. W., Eisenberger, M. A., Chan, D. W., Pearson, J. D., & Walsh, P. C. (1999). Natural history of progression after PSA elevation following radical prostatectomy. JAMA, 281(17), 1591–1597.
Flavin, R., Zadra, G., & Loda, M. (2011). Metabolic alterations and targeted therapies in prostate cancer. The Journal of Pathology, 223(2), 283–294. https://doi.org/10.1002/path.2809
Cao, Y., & Ma, J. (2011). Body mass index, prostate cancer–specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prevention Research, 4(4), 486–501. https://doi.org/10.1158/1940-6207.capr-10-0229
Freedland, S. J., Terris, M. K., Presti, J. C., Jr., Amling, C. L., Kane, C. J., Trock, B., et al. (2004). Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. Journal of Urology, 172(2), 520–524. https://doi.org/10.1097/01.ju.0000135302.58378.ae
Campeggi, A., Xylinas, E., Ploussard, G., Ouzaid, I., Fabre, A., Allory, Y., et al. (2012). Impact of body mass index on perioperative morbidity, oncological, and functional outcomes after extraperitoneal laparoscopic radical prostatectomy. Urology, 80(3), 576–584. https://doi.org/10.1016/j.urology.2012.04.066
Haque, R., Van Den Eeden, S. K., Wallner, L. P., Richert-Boe, K., Kallakury, B., Wang, R., et al. (2014). Association of body mass index and prostate cancer mortality. Obesity Research & Clinical Practice, 8(4), e374-381. https://doi.org/10.1016/j.orcp.2013.06.002
Joshu, C. E., Mondul, A. M., Menke, A., Meinhold, C., Han, M., Humphreys, E. B., et al. (2011). Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prevention Research (Philadelphia, Pa.), 4(4), 544–551. https://doi.org/10.1158/1940-6207.capr-10-0257
Whitley, B. M., Moreira, D. M., Thomas, J. A., Aronson, W. J., Terris, M. K., Presti, J. C., Jr., et al. (2011). Preoperative weight change and risk of adverse outcome following radical prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital database. Prostate Cancer and Prostatic Diseases, 14(4), 361–366. https://doi.org/10.1038/pcan.2011.42
Chen, Q., Chen, T., Shi, W., Zhang, T., Zhang, W., Jin, Z., et al. (2016). Adult weight gain and risk of prostate cancer: A dose-response meta-analysis of observational studies. International Journal of Cancer, 138(4), 866–874. https://doi.org/10.1002/ijc.29846
Bonn, S. E., Wiklund, F., Sjolander, A., Szulkin, R., Stattin, P., Holmberg, E., et al. (2014). Body mass index and weight change in men with prostate cancer: Progression and mortality. Cancer Causes and Control, 25(8), 933–943. https://doi.org/10.1007/s10552-014-0393-3
Troeschel, A. N., Hartman, T. J., Jacobs, E. J., Stevens, V. L., Gansler, T., Flanders, W. D., et al. (2020). Postdiagnosis body mass index, weight change, and mortality from prostate cancer, cardiovascular disease, and all causes among survivors of nonmetastatic prostate cancer. Journal of Clinical Oncology, 38(18), 2018–2027. https://doi.org/10.1200/JCO.19.02185
Allott, E. H., Masko, E. M., & Freedland, S. J. (2013). Obesity and prostate cancer: Weighing the evidence. European Urology, 63(5), 800–809. https://doi.org/10.1016/j.eururo.2012.11.013
Spitz, M. R., Strom, S. S., Yamamura, Y., Troncoso, P., Babaian, R. J., Scardino, P. T., et al. (2000). Epidemiologic determinants of clinically relevant prostate cancer. International Journal of Cancer, 89(3), 259–264.
Eheman, C., Henley, S. J., Ballard-Barbash, R., Jacobs, E. J., Schymura, M. J., Noone, A. M., et al. (2012). Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer, 118(9), 2338–2366. https://doi.org/10.1002/cncr.27514
Zadra, G., Photopoulos, C., & Loda, M. (2013). The fat side of prostate cancer. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids, 1831(10), 1518–1532. https://doi.org/10.1016/J.Bbalip.2013.03.010
Blando, J., Saha, A., Kiguchi, K., & DiGiovanni, J. (2013). Obesity, inflammation and prostate cancer. In A. J. Dannenberg, & N. A. Berger (Eds.), Obesity, inflammation and cancer (Vol. 7, pp. 235–256, Energy Balance and Cancer, Vol. 7). Springer.
De Nunzio, C., Albisinni, S., Freedland, S. J., Miano, L., Cindolo, L., Finazzi Agro, E., et al. (2013). Abdominal obesity as risk factor for prostate cancer diagnosis and high grade disease: A prospective multicenter Italian cohort study. Urologic Oncology, 31(7), 997–1002. https://doi.org/10.1016/j.urolonc.2011.08.007
van Kruijsdijk, R. C., van der Wall, E., & Visseren, F. L. (2009). Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiology, Biomarkers & Prevention, 18(10), 2569–2578. https://doi.org/10.1158/1055-9965.EPI-09-0372
Lysaght, J., van der Stok, E. P., Allott, E. H., Casey, R., Donohoe, C. L., Howard, J. M., et al. (2011). Pro-inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer Letters, 312(1), 62–72. https://doi.org/10.1016/j.canlet.2011.07.034
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–867. https://doi.org/10.1038/nature01322
Cowey, S., & Hardy, R. W. (2006). The metabolic syndrome: A high-risk state for cancer? American Journal of Pathology, 169(5), 1505–1522. https://doi.org/10.2353/ajpath.2006.051090
Park, J., Euhus, D. M., & Scherer, P. E. (2011). Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocrine Reviews, 32(4), 550–570. https://doi.org/10.1210/er.2010-0030
Baillargeon, J., & Rose, D. P. (2006). Obesity, adipokines, and prostate cancer (review). International Journal of Oncology, 28(3), 737–745.
Trayhurn, P., & Wood, I. S. (2004). Adipokines: Inflammation and the pleiotropic role of white adipose tissue. British Journal of Nutrition, 92(3), 347–355.
Ouchi, N., Parker, J. L., Lugus, J. J., & Walsh, K. (2011). Adipokines in inflammation and metabolic disease. Nature Reviews Immunology, 11(2), 85–97. https://doi.org/10.1038/nri2921
Khandekar, M. J., Cohen, P., & Spiegelman, B. M. (2011). Molecular mechanisms of cancer development in obesity. Nature Reviews Cancer, 11(12), 886–895. https://doi.org/10.1038/nrc3174
Roberts, D. L., Dive, C., & Renehan, A. G. (2010). Biological mechanisms linking obesity and cancer risk: New perspectives. Annual Review of Medicine, 61, 301–316. https://doi.org/10.1146/annurev.med.080708.082713
Grossmann, M. E., Ray, A., Nkhata, K. J., Malakhov, D. A., Rogozina, O. P., Dogan, S., et al. (2010). Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer and Metastasis Reviews, 29(4), 641–653. https://doi.org/10.1007/s10555-010-9252-1
Johnson, A. R., Milner, J. J., & Makowski, L. (2012). The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunological Reviews, 249(1), 218–238. https://doi.org/10.1111/j.1600-065X.2012.01151.x
Weisberg, S. P., McCann, D., Desai, M., Rosenbaum, M., Leibel, R. L., & Ferrante, A. W., Jr. (2003). Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation, 112(12), 1796–1808. https://doi.org/10.1172/JCI19246
Lengyel, E., Makowski, L., DiGiovanni, J., & Kolonin, M. G. (2018). Cancer as a matter of fat: The crosstalk between adipose tissue and tumors. Trends in Cancer, 4(5), 374–384. https://doi.org/10.1016/j.trecan.2018.03.004
Laurent, V., Guerard, A., Mazerolles, C., Le Gonidec, S., Toulet, A., Nieto, L., et al. (2016). Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. [Research Support, Non-U.S. Gov’t]. Nature Communications, 7, 10230. https://doi.org/10.1038/ncomms10230
Palm, W., & Thompson, C. B. (2017). Nutrient acquisition strategies of mammalian cells. Nature, 546(7657), 234–242. https://doi.org/10.1038/nature22379
Ye, H., Adane, B., Khan, N., Sullivan, T., Minhajuddin, M., Gasparetto, M., et al. (2016). Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell Stem Cell, 19(1), 23–37. https://doi.org/10.1016/j.stem.2016.06.001
Ribeiro, R., Monteiro, C., Silvestre, R., Castela, A., Coutinho, H., Fraga, A., et al. (2012). Human periprostatic white adipose tissue is rich in stromal progenitor cells and a potential source of prostate tumor stroma. [Research Support, Non-U.S. Gov’t]. Experimental Biology and Medicine, 237(10), 1155–1162. https://doi.org/10.1258/ebm.2012.012131
van Roermund, J. G., Hinnen, K. A., Tolman, C. J., Bol, G. H., Witjes, J. A., Bosch, J. L., et al. (2011). Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU International, 107(11), 1775–1779. https://doi.org/10.1111/j.1464-410X.2010.09811.x
Toren, P., & Venkateswaran, V. (2014). Periprostatic adipose tissue and prostate cancer progression: New insights into the tumor microenvironment. Clinical Genitourinary Cancer, 12(1), 21–26. https://doi.org/10.1016/j.clgc.2013.07.013
Finley, D. S., Calvert, V. S., Inokuchi, J., Lau, A., Narula, N., Petricoin, E. F., et al. (2009). Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. Journal of Urology, 182(4), 1621–1627. https://doi.org/10.1016/j.juro.2009.06.015
Nassar, Z. D., Aref, A. T., Miladinovic, D., Mah, C. Y., Raj, G. V., Hoy, A. J., et al. (2018). Peri-prostatic adipose tissue: The metabolic microenvironment of prostate cancer. BJU International, 121(Suppl 3), 9–21. https://doi.org/10.1111/bju.14173
Walz, J., Burnett, A. L., Costello, A. J., Eastham, J. A., Graefen, M., Guillonneau, B., et al. (2010). A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. European Urology, 57(2), 179–192. https://doi.org/10.1016/j.eururo.2009.11.009
Sung, M. T., Eble, J. N., & Cheng, L. (2006). Invasion of fat justifies assignment of stage pT3a in prostatic adenocarcinoma. Pathology, 38(4), 309–311. https://doi.org/10.1080/00313020600820914
Xie, H., Li, L., Zhu, G., Dang, Q., Ma, Z., He, D., et al. (2016). Correction: Infiltrated pre-adipocytes increase prostate cancer metastasis via modulation of the miR-301a/androgen receptor (AR)/TGF-beta1/Smad/MMP9 signals. Oncotarget, 7(50), 83829–83830. https://doi.org/10.18632/oncotarget.13913
Ribeiro, R., Monteiro, C., Silvestre, R., Castela, A., Coutinho, H., Fraga, A., et al. (2012). Human periprostatic white adipose tissue is rich in stromal progenitor cells and a potential source of prostate tumor stroma. Experimental Biology and Medicine (Maywood, N.J.), 237(10), 1155–1162. https://doi.org/10.1258/ebm.2012.012131
Ribeiro, R., Monteiro, C., Cunha, V., Oliveira, M. J., Freitas, M., Fraga, A., et al. (2012). Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. Journal of Experimental & Clinical Cancer Research, 31, 32. https://doi.org/10.1186/1756-9966-31-32
Ribeiro, R. J., Monteiro, C. P., Cunha, V. F., Azevedo, A. S., Oliveira, M. J., Monteiro, R., et al. (2012). Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cellular Physiology and Biochemistry, 29(1–2), 233–240. https://doi.org/10.1159/000337604
Takeda, K., Sowa, Y., Nishino, K., Itoh, K., & Fushiki, S. (2015). Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Annals of Plastic Surgery, 74(6), 728–736. https://doi.org/10.1097/SAP.0000000000000084
Zhang, Y., Daquinag, A. C., Amaya-Manzanares, F., Sirin, O., Tseng, C., & Kolonin, M. G. (2012). Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Research, 72(20), 5198–5208. https://doi.org/10.1158/0008-5472.CAN-12-0294
Himbert, C., Delphan, M., Scherer, D., Bowers, L. W., Hursting, S., & Ulrich, C. M. (2017). Signals from the adipose microenvironment and the obesity-cancer link-A systematic review. Cancer Prevention Research (Philadelphia, Pa.), 10(9), 494–506. https://doi.org/10.1158/1940-6207.CAPR-16-0322
Ribeiro, R., Monteiro, C., Catalan, V., Hu, P., Cunha, V., Rodriguez, A., et al. (2012). Obesity and prostate cancer: Gene expression signature of human periprostatic adipose tissue. BMC Medicine, 10, 108. https://doi.org/10.1186/1741-7015-10-108
Kolonin, M. G., Evans, K. W., Mani, S. A., & Gomer, R. H. (2012). Alternative origins of stroma in normal organs and disease. Stem Cell Res., 8(2), 312–323. https://doi.org/10.1016/j.scr.2011.11.005
Bellows, C. F., Zhang, Y., Chen, J., Frazier, M. L., & Kolonin, M. G. (2011). Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiology, Biomarkers & Prevention, 20(11), 2461–2468.
Bellows, C. F., Zhang, Y., Simmons, P. J., Khalsa, A. S., & Kolonin, M. G. (2011). Influence of BMI on level of circulating progenitor cells. Obesity, 19(8), 1722–1726. https://doi.org/10.1038/oby.2010.347
Zhang, Y., Daquinag, A., Traktuev, D. O., Amaya, F., Simmons, P. J., March, K. L., et al. (2009). White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Research, 69(12), 5259–5266.
Klopp, A. H., Zhang, Y., Solley, T., Amaya-Manzanares, F., Marini, F., Andreeff, M., et al. (2012). Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clinical Cancer Research, 18(3), 771–782. https://doi.org/10.1158/1078-0432.CCR-11-1916
Sirin, O., & Kolonin, M. G. (2013). Treatment of obesity as a potential complementary approach to cancer therapy. Drug Discovery Today, 11(12), 567–573.
Zhang, T., Tseng, C., Zhang, Y., Sirin, O., Corn, P. G., Li-Ning-Tapia, E. M., et al. (2016). CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nature Communications, 7, 11674. https://doi.org/10.1038/ncomms11674
Peng, Y. C., Levine, C. M., Zahid, S., Wilson, E. L., & Joyner, A. L. (2013). Sonic hedgehog signals to multiple prostate stromal stem cells that replenish distinct stromal subtypes during regeneration. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Proceedings of the National Academy of Sciences, 110(51), 20611–20616. https://doi.org/10.1073/pnas.1315729110
Zhang, Y., & Kolonin, M. G. (2016). Cytokine signaling regulating adipose stromal cell trafficking. Adipocyte, 5(4), 369–374. https://doi.org/10.1080/21623945.2016.1220452
Kaplan, J. L., Marshall, M. A., McSkimming, C. C., Harmon, D. B., Garmey, J. C., Oldham, S. N., et al. (2015). Adipocyte progenitor cells initiate monocyte chemoattractant protein-1-mediated macrophage accumulation in visceral adipose tissue. Molecular Metabolism, 4(11), 779–794. https://doi.org/10.1016/j.molmet.2015.07.010
Murdoch, C., Muthana, M., Coffelt, S. B., & Lewis, C. E. (2008). The role of myeloid cells in the promotion of tumour angiogenesis. Nature Reviews Cancer, 8(8), 618–631. https://doi.org/10.1038/nrc2444
Iyengar, N. M., Brown, K. A., Zhou, X. K., Gucalp, A., Subbaramaiah, K., Giri, D. D., et al. (2017). Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prevention Research, 10(4), 235–243. https://doi.org/10.1158/1940-6207.CAPR-16-0314
Hanahan, D., & Coussens, L. M. (2012). Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Lu, P., Weaver, V. M., & Werb, Z. (2012). The extracellular matrix: A dynamic niche in cancer progression. Journal of Cell Biology, 196(4), 395–406. https://doi.org/10.1083/jcb.201102147
Park, J., Morley, T. S., Kim, M., Clegg, D. J., & Scherer, P. E. (2014). Obesity and cancer--mechanisms underlying tumour progression and recurrence. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Review]. Nature Reviews Endocrinology, 10(8), 455–465. https://doi.org/10.1038/nrendo.2014.94
Kidd, S., Spaeth, E., Watson, K., Burks, J., Lu, H., Klopp, A., et al. (2012). Origins of the tumor microenvironment: Quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE, 7(2), e30563. https://doi.org/10.1371/journal.pone.0030563
Orecchioni, S., Gregato, G., Martin-Padura, I., Reggiani, F., Braidotti, P., Mancuso, P., et al. (2013). Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. [Research Support, Non-U.S. Gov’t]. Cancer Research, 73(19), 5880–5891. https://doi.org/10.1158/0008-5472.CAN-13-0821
Rowan, B. G., Gimble, J. M., Sheng, M., Anbalagan, M., Jones, R. K., Frazier, T. P., et al. (2014). Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS ONE, 9(2), e89595. https://doi.org/10.1371/journal.pone.0089595
Martin-Padura, I., Gregato, G., Marighetti, P., Mancuso, P., Calleri, A., Corsini, C., et al. (2012). The white adipose tissue used in lipotransfer procedures is a rich reservoir of CD34+ progenitors able to promote cancer progression. Cancer Research, 72(1), 325–334. https://doi.org/10.1158/0008-5472.CAN-11-1739
Zhao, M., Dumur, C. I., Holt, S. E., Beckman, M. J., & Elmore, L. W. (2010). Multipotent adipose stromal cells and breast cancer development: Think globally, act locally. Molecular Carcinogenesis, 49(11), 923–927. https://doi.org/10.1002/mc.20675
Picon-Ruiz, M., Pan, C., Drews-Elger, K., Jang, K., Besser, A. H., Zhao, D., et al. (2016). Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Research, 76(2), 491–504. https://doi.org/10.1158/0008-5472.CAN-15-0927
Nowicka, A., Marini, F. C., Solley, T. N., Elizondo, P. B., Zhang, Y., Sharp, H. J., et al. (2013). Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS ONE, 8(12), e81859. https://doi.org/10.1371/journal.pone.0081859
Salimian Rizi, B., Caneba, C., Nowicka, A., Nabiyar, A. W., Liu, X., Chen, K., et al. (2015). Nitric oxide mediates metabolic coupling of omentum-derived adipose stroma to ovarian and endometrial cancer cells. [Research Support, Non-U.S. Gov’t]. Cancer Research, 75(2), 456–4571. https://doi.org/10.1158/0008-5472.CAN-14-1337
Duong, M. N., Cleret, A., Matera, E. L., Chettab, K., Mathe, D., Valsesia-Wittmann, S., et al. (2015). Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Research, 17, 57–63. https://doi.org/10.1186/s13058-015-0569-0
Houthuijzen, J. M., Daenen, L. G., Roodhart, J. M., & Voest, E. E. (2012). The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. [Review]. British Journal of Cancer, 106(12), 1901–1906. https://doi.org/10.1038/bjc.2012.201
Su, F., Ahn, S., Saha, A., DiGiovanni, J., & Kolonin, M. G. (2019). Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene, 38(11), 1979–1988. https://doi.org/10.1038/s41388-018-0558-8
Giovannucci, E., & Michaud, D. (2007). The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology, 132(6), 2208–2225. https://doi.org/10.1053/j.gastro.2007.03.050
Giovannucci, E., Rimm, E. B., Colditz, G. A., Stampfer, M. J., Ascherio, A., Chute, C. C., et al. (1993). A prospective study of dietary fat and risk of prostate cancer. Journal of the National Cancer Institute, 85(19), 1571–1579.
Freedland, S. J., & Aronson, W. J. (2004). Examining the relationship between obesity and prostate cancer. Revista de Urología, 6(2), 73–81.
Strom, S. S., Wang, X., Pettaway, C. A., Logothetis, C. J., Yamamura, Y., Do, K. A., et al. (2005). Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clinical Cancer Research, 11(19 Pt 1), 6889–6894.
Frasca, F., Pandini, G., Sciacca, L., Pezzino, V., Squatrito, S., Belfiore, A., et al. (2008). The role of insulin receptors and IGF-I receptors in cancer and other diseases. Archives of Physiology and Biochemistry, 114(1), 23–37. https://doi.org/10.1080/13813450801969715
Blando, J., Moore, T., Hursting, S., Jiang, G., Saha, A., Beltran, L., et al. (2011). Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Cancer Prevention Research, 4(12), 2002–2014. https://doi.org/10.1158/1940-6207.CAPR-11-0182
Saha, A., Ahn, S., Blando, J., Su, F., Kolonin, M. G., & DiGiovanni, J. (2017). Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives Myc-induced prostate cancer in obese mice. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-17-0284
Rossi, E. L., Khatib, S. A., Doerstling, S. S., Bowers, L. W., Pruski, M., Ford, N. A., et al. (2018). Resveratrol inhibits obesity-associated adipose tissue dysfunction and tumor growth in a mouse model of postmenopausal claudin-low breast cancer. Molecular Carcinogenesis, 57(3), 393–407. https://doi.org/10.1002/mc.22763
Checkley, L. A., Rho, O., Angel, J. M., Cho, J., Blando, J., Beltran, L., et al. (2014). Metformin inhibits skin tumor promotion in overweight and obese mice. Cancer Prevention Research (Philadelphia, Pa.), 7(1), 54–64. https://doi.org/10.1158/1940-6207.CAPR-13-0110
Moore, T., Beltran, L., Carbajal, S., Hursting, S. D., & DiGiovanni, J. (2012). Energy balance modulates mouse skin tumor promotion through altered IGF-1R and EGFR crosstalk. Cancer Prevention Research (Philadelphia, Pa.), 5(10), 1236–1246. https://doi.org/10.1158/1940-6207.CAPR-12-0234
Aiuti, A., Webb, I. J., Bleul, C., Springer, T., & Gutierrez-Ramos, J. C. (1997). The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. Journal of Experimental Medicine, 185(1), 111–120.
Secchiero, P., Celeghini, C., Cutroneo, G., Di Baldassarre, A., Rana, R., & Zauli, G. (2000). Differential effects of stromal derived factor-1 alpha (SDF-1 alpha) on early and late stages of human megakaryocytic development. Anatomical Record, 260(2), 141–147.
Hattermann, K., & Mentlein, R. (2013). An infernal trio: The chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. [Review]. Annals of Anatomy, 195(2), 103–110. https://doi.org/10.1016/j.aanat.2012.10.013
Conley-LaComb, M. K., Saliganan, A., Kandagatla, P., Chen, Y. Q., Cher, M. L., & Chinni, S. R. (2013). PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Molecular Cancer, 12(1), 85. https://doi.org/10.1186/1476-4598-12-85
Rivat, C., Sebaihi, S., Van Steenwinckel, J., Fouquet, S., Kitabgi, P., Pohl, M., et al. (2014). Src family kinases involved in CXCL12-induced loss of acute morphine analgesia. Brain, Behavior, and Immunity, 38, 38–52. https://doi.org/10.1016/j.bbi.2013.11.010
Sun, X., Cheng, G., Hao, M., Zheng, J., Zhou, X., Zhang, J., et al. (2010). CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. Cancer and Metastasis Reviews, 29(4), 709–722. https://doi.org/10.1007/s10555-010-9256-x
Duda, D. G., Kozin, S. V., Kirkpatrick, N. D., Xu, L., Fukumura, D., & Jain, R. K. (2011). CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: An emerging sensitizer for anticancer therapies? Clinical Cancer Research, 17(8), 2074–2080. https://doi.org/10.1158/1078-0432.CCR-10-2636
Decaillot, F. M., Kazmi, M. A., Lin, Y., Ray-Saha, S., Sakmar, T. P., & Sachdev, P. (2011). CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. Journal of Biological Chemistry, 286(37), 32188–32197. https://doi.org/10.1074/jbc.M111.277038
Levoye, A., Balabanian, K., Baleux, F., Bachelerie, F., & Lagane, B. (2009). CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. [Research Support, Non-U.S. Gov’t]. Blood, 113(24), 6085–6093. https://doi.org/10.1182/blood-2008-12-196618
Luker, K. E., Gupta, M., & Luker, G. D. (2009). Imaging chemokine receptor dimerization with firefly luciferase complementation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. The FASEB Journal, 23(3), 823–834. https://doi.org/10.1096/fj.08-116749
Zhao, H. B., Tang, C. L., Hou, Y. L., Xue, L. R., Li, M. Q., Du, M. R., et al. (2012). CXCL12/CXCR4 axis triggers the activation of EGF receptor and ERK signaling pathway in CsA-induced proliferation of human trophoblast cells. [Clinical Trial Research Support, Non-U.S. Gov’t]. PLoS One, 7(7), e38375. https://doi.org/10.1371/journal.pone.0038375
McGinn, O. J., Marinov, G., Sawan, S., & Stern, P. L. (2012). CXCL12 receptor preference, signal transduction, biological response and the expression of 5T4 oncofoetal glycoprotein. [Research Support, Non-U.S. Gov’t]. Journal of Cell Science, 125(Pt 22), 5467–5478. https://doi.org/10.1242/jcs.109488
Wang, J., Shiozawa, Y., Wang, J., Wang, Y., Jung, Y., Pienta, K. J., et al. (2008). The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.]. Journal of Biological Chemistry, 283(7), 4283–4294. https://doi.org/10.1074/jbc.M707465200
Akashi, T., Koizumi, K., Tsuneyama, K., Saiki, I., Takano, Y., & Fuse, H. (2008). Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Science, 99(3), 539–542. https://doi.org/10.1111/j.1349-7006.2007.00712.x
Sun, Y. X., Wang, J., Shelburne, C. E., Lopatin, D. E., Chinnaiyan, A. M., Rubin, M. A., et al. (2003). Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. Journal of Cellular Biochemistry, 89(3), 462–473. https://doi.org/10.1002/jcb.10522
Mochizuki, H., Matsubara, A., Teishima, J., Mutaguchi, K., Yasumoto, H., Dahiya, R., et al. (2004). Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: A possible predictor of metastasis. Biochemical and Biophysical Research Communications, 320(3), 656–663. https://doi.org/10.1016/j.bbrc.2004.06.013
Jung, S. J., Kim, C. I., Park, C. H., Chang, H. S., Kim, B. H., Choi, M. S., et al. (2011). Correlation between chemokine receptor CXCR4 expression and prognostic factors in patients with prostate cancer. Korean Journal of Urology, 52(9), 607–611. https://doi.org/10.4111/kju.2011.52.9.607
Sun, Y. X., Schneider, A., Jung, Y., Wang, J., Dai, J., Wang, J., et al. (2005). Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. Journal of Bone and Mineral Research, 20(2), 318–329. https://doi.org/10.1359/JBMR.041109
Cawthorn, W. P., Scheller, E. L., Learman, B. S., Parlee, S. D., Simon, B. R., Mori, H., et al. (2014). Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metabolism, 20(2), 368–375. https://doi.org/10.1016/j.cmet.2014.06.003
Devlin, M. J., Cloutier, A. M., Thomas, N. A., Panus, D. A., Lotinun, S., Pinz, I., et al. (2010). Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. Journal of Bone and Mineral Research, 25(9), 2078–2088. https://doi.org/10.1002/jbmr.82
Bredella, M. A., Fazeli, P. K., Miller, K. K., Misra, M., Torriani, M., Thomas, B. J., et al. (2009). Increased bone marrow fat in anorexia nervosa. Journal of Clinical Endocrinology and Metabolism, 94(6), 2129–2136. https://doi.org/10.1210/jc.2008-2532
Morris, E. V., & Edwards, C. M. (2016). Bone marrow adipose tissue: A new player in cancer metastasis to bone. Frontiers in Endocrinology (Lausanne), 7, 90. https://doi.org/10.3389/fendo.2016.00090
Herroon, M. K., Rajagurubandara, E., Hardaway, A. L., Powell, K., Turchick, A., Feldmann, D., et al. (2013). Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget, 4(11), 2108–2123. https://doi.org/10.18632/oncotarget.1482
Templeton, Z. S., Lie, W. R., Wang, W., Rosenberg-Hasson, Y., Alluri, R. V., Tamaresis, J. S., et al. (2015). Breast cancer cell colonization of the human bone marrow adipose tissue niche. Neoplasia, 17(12), 849–861. https://doi.org/10.1016/j.neo.2015.11.005
Hardaway, A. L., Herroon, M. K., Rajagurubandara, E., & Podgorski, I. (2015). Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clinical & Experimental Metastasis, 32(4), 353–368. https://doi.org/10.1007/s10585-015-9714-5
Matsushita, Y., Chu, A. K. Y., Ono, W., Welch, J. D., & Ono, N. (2021). Intercellular interactions of an adipogenic CXCL12-expressing stromal cell subset in murine bone marrow. Journal of Bone and Mineral Research, 36(6), 1145–1158. https://doi.org/10.1002/jbmr.4282
Hebert, C. A., & Baker, J. B. (1993). Interleukin-8: A review. Cancer Investigation, 11(6), 743–750. https://doi.org/10.3109/07357909309046949
Holmes, W. E., Lee, J., Kuang, W. J., Rice, G. C., & Wood, W. I. (2009). Structure and functional expression of a human interleukin-8 receptor. Science. 1991. 253: 1278–1280. Journal of Immunology, 183(5), 2895–2897
Morohashi, H., Miyawaki, T., Nomura, H., Kuno, K., Murakami, S., Matsushima, K., et al. (1995). Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. Journal of Leukocyte Biology, 57(1), 180–187. https://doi.org/10.1002/jlb.57.1.180
Kim, S. J., Uehara, H., Karashima, T., McCarty, M., Shih, N., & Fidler, I. J. (2001). Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia, 3(1), 33–42. https://doi.org/10.1038/sj.neo.7900124
Miyake, M., Lawton, A., Goodison, S., Urquidi, V., & Rosser, C. J. (2014). Chemokine (C-X-C motif) ligand 1 (CXCL1) protein expression is increased in high-grade prostate cancer. Pathology, Research and Practice, 210(2), 74–78. https://doi.org/10.1016/j.prp.2013.08.013
Straczkowski, M., Dzienis-Straczkowska, S., Stepien, A., Kowalska, I., Szelachowska, M., & Kinalska, I. (2002). Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. Journal of Clinical Endocrinology and Metabolism, 87(10), 4602–4606. https://doi.org/10.1210/jc.2002-020135
Wald, O., Shapira, O. M., & Izhar, U. (2013). CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics, 3(1), 26–33. https://doi.org/10.7150/thno.4922
Brat, D. J., Bellail, A. C., & Van Meir, E. G. (2005). The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-Oncology, 7(2), 122–133. https://doi.org/10.1215/S1152851704001061
Wu, D., LaRosa, G. J., & Simon, M. I. (1993). G protein-coupled signal transduction pathways for interleukin-8. Science, 261(5117), 101–103. https://doi.org/10.1126/science.8316840
Wu, Y., Wang, S., Farooq, S. M., Castelvetere, M. P., Hou, Y., Gao, J. L., et al. (2012). A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. Journal of Biological Chemistry, 287(8), 5744–5755. https://doi.org/10.1074/jbc.M111.315762
Fuhler, G. M., Knol, G. J., Drayer, A. L., & Vellenga, E. (2005). Impaired interleukin-8- and GROalpha-induced phosphorylation of extracellular signal-regulated kinase result in decreased migration of neutrophils from patients with myelodysplasia. Journal of Leukocyte Biology, 77(2), 257–266. https://doi.org/10.1189/jlb.0504306
Knall, C., Worthen, G. S., & Johnson, G. L. (1997). Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proceedings of the National Academy of Sciences, 94(7), 3052–3057. https://doi.org/10.1073/pnas.94.7.3052
Thelen, M., Uguccioni, M., & Bosiger, J. (1995). PI 3-kinase-dependent and independent chemotaxis of human neutrophil leukocytes. Biochemical and Biophysical Research Communications, 217(3), 1255–1262. https://doi.org/10.1006/bbrc.1995.2903
Waugh, D. J., & Wilson, C. (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741. https://doi.org/10.1158/1078-0432.CCR-07-4843
Chavey, C., Lazennec, G., Lagarrigue, S., Clape, C., Iankova, I., Teyssier, J., et al. (2009). CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metabolism, 9(4), 339–349. https://doi.org/10.1016/j.cmet.2009.03.002
Begley, L. A., Kasina, S., Mehra, R., Adsule, S., Admon, A. J., Lonigro, R. J., et al. (2008). CXCL5 promotes prostate cancer progression. Neoplasia, 10(3), 244–254. https://doi.org/10.1593/neo.07976
Qi, Y., Zhao, W., Li, M., Shao, M., Wang, J., Sui, H., et al. (2018). High C-X-C motif chemokine 5 expression is associated with malignant phenotypes of prostate cancer cells via autocrine and paracrine pathways. International Journal of Oncology, 53(1), 358–370. https://doi.org/10.3892/ijo.2018.4388
Ammirante, M., Shalapour, S., Kang, Y., Jamieson, C. A., & Karin, M. (2014). Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proceedings of the National Academy of Sciences, 111(41), 14776–14781. https://doi.org/10.1073/pnas.1416498111
Kusuyama, J., Bandow, K., Ohnishi, T., Amir, M. S., Shima, K., Semba, I., et al. (2019). CXCL13 is a differentiation- and hypoxia-induced adipocytokine that exacerbates the inflammatory phenotype of adipocytes through PHLPP1 induction. The Biochemical Journal, 476(22), 3533–3548. https://doi.org/10.1042/BCJ20190709
Kabir, S. M., Lee, E. S., & Son, D. S. (2014). Chemokine network during adipogenesis in 3T3-L1 cells: Differential response between growth and proinflammatory factor in preadipocytes vs. adipocytes. Adipocyte, 3(2), 97–106. https://doi.org/10.4161/adip.28110
El Haibi, C. P., Sharma, P. K., Singh, R., Johnson, P. R., Suttles, J., Singh, S., et al. (2010). PI3Kp110-, Src-, FAK-dependent and DOCK2-independent migration and invasion of CXCL13-stimulated prostate cancer cells. Molecular Cancer, 9, 85. https://doi.org/10.1186/1476-4598-9-85
El-Haibi, C. P., Singh, R., Sharma, P. K., Singh, S., & Lillard, J. W., Jr. (2011). CXCL13 mediates prostate cancer cell proliferation through JNK signalling and invasion through ERK activation. Cell Proliferation, 44(4), 311–319. https://doi.org/10.1111/j.1365-2184.2011.00757.x
El-Haibi, C. P., Sharma, P., Singh, R., Gupta, P., Taub, D. D., Singh, S., et al. (2013). Differential G protein subunit expression by prostate cancer cells and their interaction with CXCR5. Molecular Cancer, 12, 64. https://doi.org/10.1186/1476-4598-12-64
Hattermann, K., Bartsch, K., Gebhardt, H. H., Mehdorn, H. M., Synowitz, M., Schmitt, A. D., et al. (2016). “Inverse signaling” of the transmembrane chemokine CXCL16 contributes to proliferative and anti-apoptotic effects in cultured human meningioma cells. Cell Communication and Signaling: CCS, 14(1), 26. https://doi.org/10.1186/s12964-016-0149-7
Kurki, E., Shi, J., Martonen, E., Finckenberg, P., & Mervaala, E. (2012). Distinct effects of calorie restriction on adipose tissue cytokine and angiogenesis profiles in obese and lean mice. Nutrition & Metabolism (London), 9(1), 64. https://doi.org/10.1186/1743-7075-9-64
Jung, Y., Kim, J. K., Shiozawa, Y., Wang, J., Mishra, A., Joseph, J., et al. (2013). Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nature Communications, 4, 1795. https://doi.org/10.1038/ncomms2766
Kapur, N., Mir, H., Sonpavde, G. P., Jain, S., Bae, S., Lillard, J. W., Jr., et al. (2019). Prostate cancer cells hyper-activate CXCR6 signaling by cleaving CXCL16 to overcome effect of docetaxel. Cancer Letters, 454, 1–13. https://doi.org/10.1016/j.canlet.2019.04.001
Kanda, H., Tateya, S., Tamori, Y., Kotani, K., Hiasa, K., Kitazawa, R., et al. (2006). MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of Clinical Investigation, 116(6), 1494–1505. https://doi.org/10.1172/JCI26498
Sartipy, P., & Loskutoff, D. J. (2003). Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proceedings of the National Academy of Sciences, 100(12), 7265–7270. https://doi.org/10.1073/pnas.1133870100
Gerhardt, C. C., Romero, I. A., Cancello, R., Camoin, L., & Strosberg, A. D. (2001). Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Molecular and Cellular Endocrinology, 175(1–2), 81–92. https://doi.org/10.1016/s0303-7207(01)00394-x
Tsaur, I., Noack, A., Makarevic, J., Oppermann, E., Waaga-Gasser, A. M., Gasser, M., et al. (2015). CCL2 chemokine as a potential biomarker for prostate cancer: A pilot study. Cancer Research and Treatment, 47(2), 306–312. https://doi.org/10.4143/crt.2014.015
Gschwandtner, M., Derler, R., & Midwood, K. S. (2019). More than just attractive: How CCL2 influences myeloid cell behavior beyond chemotaxis. Frontiers in Immunology, 10, 2759. https://doi.org/10.3389/fimmu.2019.02759
Christiansen, T., Richelsen, B., & Bruun, J. M. (2005). Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. International Journal of Obesity, 29(1), 146–150. https://doi.org/10.1038/sj.ijo.0802839
Huang, M., Narita, S., Numakura, K., Tsuruta, H., Saito, M., Inoue, T., et al. (2012). A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate, 72(16), 1779–1788. https://doi.org/10.1002/pros.22531
Loberg, R. D., Ying, C., Craig, M., Yan, L., Snyder, L. A., & Pienta, K. J. (2007). CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia, 9(7), 556–562. https://doi.org/10.1593/neo.07307
Mathews, J. A., Wurmbrand, A. P., Ribeiro, L., Neto, F. L., & Shore, S. A. (2014). Induction of IL-17A precedes development of airway hyperresponsiveness during diet-induced obesity and correlates with complement factor D. Frontiers in Immunology, 5, 440. https://doi.org/10.3389/fimmu.2014.00440
Xu, H., Barnes, G. T., Yang, Q., Tan, G., Yang, D., Chou, C. J., et al. (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of Clinical Investigation, 112(12), 1821–1830. https://doi.org/10.1172/JCI19451
Fang, L. Y., Izumi, K., Lai, K. P., Liang, L., Li, L., Miyamoto, H., et al. (2013). Infiltrating macrophages promote prostate tumorigenesis via modulating androgen receptor-mediated CCL4-STAT3 signaling. Cancer Research, 73(18), 5633–5646. https://doi.org/10.1158/0008-5472.CAN-12-3228
Huang, R., Wang, S., Wang, N., Zheng, Y., Zhou, J., Yang, B., et al. (2020). CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating beta-catenin/STAT3 signaling. Cell Death & Disease, 11(4), 234. https://doi.org/10.1038/s41419-020-2435-y
Liu, Y., Cai, Y., Liu, L., Wu, Y., & Xiong, X. (2018). Crucial biological functions of CCL7 in cancer. PeerJ, 6, e4928. https://doi.org/10.7717/peerj.4928
Van Damme, J., Proost, P., Lenaerts, J. P., & Opdenakker, G. (1992). Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. Journal of Experimental Medicine, 176(1), 59–65. https://doi.org/10.1084/jem.176.1.59
Tourniaire, F., Romier-Crouzet, B., Lee, J. H., Marcotorchino, J., Gouranton, E., Salles, J., et al. (2013). Chemokine expression in inflamed adipose tissue is mainly mediated by NF-kappaB. PLoS ONE, 8(6), e66515. https://doi.org/10.1371/journal.pone.0066515
Cuesta-Gomez, N., Graham, G. J., & Campbell, J. D. M. (2021). Chemokines and their receptors: Predictors of the therapeutic potential of mesenchymal stromal cells. Journal of Translational Medicine, 19(1), 156. https://doi.org/10.1186/s12967-021-02822-5
Nagarsheth, N., Wicha, M. S., & Zou, W. (2017). Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nature Reviews Immunology, 17(9), 559–572. https://doi.org/10.1038/nri.2017.49
Ford, J., Hughson, A., Lim, K., Bardina, S. V., Lu, W., Charo, I. F., et al. (2018). CCL7 is a negative regulator of cutaneous inflammation following leishmania major infection. Frontiers in Immunology, 9, 3063. https://doi.org/10.3389/fimmu.2018.03063
Palomino, D. C., & Marti, L. C. (2015). Chemokines and immunity. Einstein (Sao Paulo), 13(3), 469–473. https://doi.org/10.1590/S1679-45082015RB3438
Zhu, F., Liu, P., Li, J., & Zhang, Y. (2014). Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. Oncology Reports, 31(5), 2049–2054. https://doi.org/10.3892/or.2014.3060
Rosen, E. D., & Spiegelman, B. M. (2014). What we talk about when we talk about fat. Cell, 156(1–2), 20–44. https://doi.org/10.1016/j.cell.2013.12.012
Ducharme, N. A., & Bickel, P. E. (2008). Lipid droplets in lipogenesis and lipolysis. Endocrinology, 149(3), 942–949.
Granneman, J. G., & Moore, H. P. (2008). Location, location: protein trafficking and lipolysis in adipocytes. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S. Review]. Trends in Endocrinology & Metabolism, 19(1), 3–9. https://doi.org/10.1016/j.tem.2007.10.006
Currie, E., Schulze, A., Zechner, R., Walther, T. C., & Farese, R. V., Jr. (2013). Cellular fatty acid metabolism and cancer. Cell Metabolism, 18(2), 153–161. https://doi.org/10.1016/j.cmet.2013.05.017
Nomura, D. K., Lombardi, D. P., Chang, J. W., Niessen, S., Ward, A. M., Long, J. Z., et al. (2011). Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chemistry & Biology, 18(7), 846–856. https://doi.org/10.1016/j.chembiol.2011.05.009
Wang, Y. Y., Attane, C., Milhas, D., Dirat, B., Dauvillier, S., Guerard, A., et al. (2017). Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight, 2(4), e87489. https://doi.org/10.1172/jci.insight.87489
Arner, P., & Langin, D. (2014). Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends in Endocrinology and Metabolism, 25(5), 255–262. https://doi.org/10.1016/j.tem.2014.03.002
Rohm, M., Schafer, M., Laurent, V., Ustunel, B. E., Niopek, K., Algire, C., et al. (2016). An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nature Medicine, 22(10), 1120–1130. https://doi.org/10.1038/nm.4171
Okumura, T., Ohuchida, K., Sada, M., Abe, T., Endo, S., Koikawa, K., et al. (2017). Extra-pancreatic invasion induces lipolytic and fibrotic changes in the adipose microenvironment, with released fatty acids enhancing the invasiveness of pancreatic cancer cells. Oncotarget, 8(11), 18280–18295. https://doi.org/10.18632/oncotarget.15430
Yamaguchi, J., Ohtani, H., Nakamura, K., Shimokawa, I., & Kanematsu, T. (2008). Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. American Journal of Clinical Pathology, 130(3), 382–388. https://doi.org/10.1309/MX6KKA1UNJ1YG8VN
Nieman, K. M., Kenny, H. A., Penicka, C. V., Ladanyi, A., Buell-Gutbrod, R., Zillhardt, M. R., et al. (2011). Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine, 17(11), 1498–1503. https://doi.org/10.1038/nm.2492
Dirat, B., Bochet, L., Dabek, M., Daviaud, D., Dauvillier, S., Majed, B., et al. (2011). Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Research, 71(7), 2455–2465. https://doi.org/10.1158/0008-5472.CAN-10-3323
Balaban, S., Shearer, R. F., Lee, L. S., van Geldermalsen, M., Schreuder, M., Shtein, H. C., et al. (2017). Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer & Metabolism, 5, 1. https://doi.org/10.1186/s40170-016-0163-7
Wen, Y. A., Xing, X., Harris, J. W., Zaytseva, Y. Y., Mitov, M. I., Napier, D. L., et al. (2017). Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death & Disease, 8(2), e2593. https://doi.org/10.1038/cddis.2017.21
Daquinag, A. C., Gao, Z., Fussell, C., Immaraj, L., Pasqualini, R., Arap, W., et al. (2021). Fatty acid mobilization from adipose tissue is mediated by CD36 posttranslational modifications and intracellular trafficking. JCI Insight, 6(17). https://doi.org/10.1172/jci.insight.147057
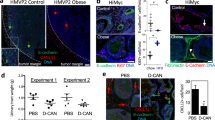
Su, F., Daquinag, A. C., Ahn, S., Saha, A., Dai, Y., Zhao, Z., et al. (2021). Progression of prostate carcinoma is promoted by adipose stromal cell-secreted CXCL12 signaling in prostate epithelium. NPJ Precision Oncology, 5(1), 26. https://doi.org/10.1038/s41698-021-00160-9
Leitner, B. P., Huang, S., Brychta, R. J., Duckworth, C. J., Baskin, A. S., McGehee, S., et al. (2017). Mapping of human brown adipose tissue in lean and obese young men. Proceedings of the National Academy of Sciences, 114(32), 8649–8654. https://doi.org/10.1073/pnas.1705287114
Alvarez-Artime, A., Garcia-Soler, B., Sainz, R. M., & Mayo, J. C. (2021). Emerging roles for browning of white adipose tissue in prostate cancer malignant behaviour. International Journal of Molecular Sciences, 22(11). https://doi.org/10.3390/ijms22115560
Collins, S. (2011). beta-Adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Frontiers in Endocrinology (Lausanne), 2, 102. https://doi.org/10.3389/fendo.2011.00102
Puigserver, P., Wu, Z., Park, C. W., Graves, R., Wright, M., & Spiegelman, B. M. (1998). A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell, 92(6), 829–839. https://doi.org/10.1016/s0092-8674(00)81410-5
Hursting, S. D., Dunlap, S. M., Ford, N. A., Hursting, M. J., & Lashinger, L. M. (2013). Calorie restriction and cancer prevention: A mechanistic perspective. Cancer & Metabolism, 1(1), 10. https://doi.org/10.1186/2049-3002-1-10
Galet, C., Gray, A., Said, J. W., Castor, B., Wan, J., Beltran, P. J., et al. (2013). Effects of calorie restriction and IGF-1 receptor blockade on the progression of 22Rv1 prostate cancer xenografts. International Journal of Molecular Sciences, 14(7), 13782–13795. https://doi.org/10.3390/ijms140713782
Freedland, S. J., Mavropoulos, J., Wang, A., Darshan, M., Demark-Wahnefried, W., Aronson, W. J., et al. (2008). Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate, 68(1), 11–19. https://doi.org/10.1002/pros.20683
Allott, E. H., & Hursting, S. D. (2015). Obesity and cancer: Mechanistic insights from transdisciplinary studies. Endocrine-Related Cancer, 22(6), R365-386. https://doi.org/10.1530/ERC-15-0400
Dorling, J. L., van Vliet, S., Huffman, K. M., Kraus, W. E., Bhapkar, M., Pieper, C. F., et al. (2020). Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: Highlights from CALERIE phase 2. Nutrition Reviews. https://doi.org/10.1093/nutrit/nuaa085
Harvie, M., Wright, C., Pegington, M., McMullan, D., Mitchell, E., Martin, B., et al. (2013). The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. British Journal of Nutrition, 110(8), 1534–1547. https://doi.org/10.1017/S0007114513000792
Harvie, M. N., Pegington, M., Mattson, M. P., Frystyk, J., Dillon, B., Evans, G., et al. (2011). The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. International Journal of Obesity, 35(5), 714–727. https://doi.org/10.1038/ijo.2010.171
Harvie, M. N., Sims, A. H., Pegington, M., Spence, K., Mitchell, A., Vaughan, A. A., et al. (2016). Intermittent energy restriction induces changes in breast gene expression and systemic metabolism. Breast Cancer Research, 18(1), 57. https://doi.org/10.1186/s13058-016-0714-4
Hamilton-Reeves, J. M. (2017-2021). Weight management aimed to reduce risk and improve outcomes from radical prostatectomy (WARRIOR). (ed.). NCT03261271
Dimachkie, M. D., Bechtel, M. D., Robertson, H. L., Michel, C., Lee, E. K., Sullivan, D. K., et al. (2021). Exploration of biomarkers from a pilot weight management study for men undergoing radical prostatectomy. Urologic Oncology. https://doi.org/10.1016/j.urolonc.2021.01.010
Hamilton-Reeves, J. M., Johnson, C. N., Hand, L. K., Bechtel, M. D., Robertson, H. L., Michel, C., et al. (2020). Feasibility of a weight management program tailored for overweight men with localized prostate cancer - A pilot study. Nutrition and Cancer, 1–16.https://doi.org/10.1080/01635581.2020.1856890
Lin, D. W., Neuhouser, M. L., Schenk, J. M., Coleman, I. M., Hawley, S., Gifford, D., et al. (2007). Low-fat, low-glycemic load diet and gene expression in human prostate epithelium: A feasibility study of using cDNA microarrays to assess the response to dietary intervention in target tissues. Cancer Epidemiology, Biomarkers & Prevention, 16(10), 2150–2154.
Henning, S. M., Galet, C., Gollapudi, K., Byrd, J. B., Liang, P., Li, Z., et al. (2018). Phase II prospective randomized trial of weight loss prior to radical prostatectomy. Prostate Cancer and Prostatic Diseases, 21(2), 212–220. https://doi.org/10.1038/s41391-017-0001-1
Demark-Wahnefried, W., Rais-Bahrami, S., Desmond, R. A., Gordetsky, J. B., Hunter, G. R., Yang, E. S., et al. (2017). Presurgical weight loss affects tumour traits and circulating biomarkers in men with prostate cancer. [Clinical Study]. British Journal of Cancer, 117, 1303. https://doi.org/10.1038/bjc.2017.303. https://www.nature.com/articles/bjc2017303#supplementary-information
Fruge, A. D., Ptacek, T., Tsuruta, Y., Morrow, C. D., Azrad, M., Desmond, R. A., et al. (2018). Dietary changes impact the gut microbe composition in overweight and obese men with prostate cancer undergoing radical prostatectomy. Journal of the Academy of Nutrition and Dietetics, 118(4), 714–723 e711. https://doi.org/10.1016/j.jand.2016.10.017
Wilson, R. L., Shannon, T., Calton, E., Galvao, D. A., Taaffe, D. R., Hart, N. H., et al. (2020). Efficacy of a weight loss program prior to robot assisted radical prostatectomy in overweight and obese men with prostate cancer. Surgical Oncology, 35, 182–188. https://doi.org/10.1016/j.suronc.2020.08.006
Varady, K. A. (2011). Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obesity Reviews, 12(7), e593-601. https://doi.org/10.1111/j.1467-789X.2011.00873.x
Ozkul, C., Yalinay, M., & Karakan, T. (2019). Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting. The Turkish Journal of Gastroenterology, 30(12), 1030–1035. https://doi.org/10.5152/tjg.2019.19185
Guo, Y., Luo, S., Ye, Y., Yin, S., Fan, J., & Xia, M. (2020). Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. Journal of Clinical Endocrinology and Metabolism. https://doi.org/10.1210/clinem/dgaa644
Johnson, J. B., Summer, W., Cutler, R. G., Martin, B., Hyun, D. H., Dixit, V. D., et al. (2007). Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biology & Medicine, 42(5), 665–674. https://doi.org/10.1016/j.freeradbiomed.2006.12.005
Schwingshackl, L., Zahringer, J., Nitschke, K., Torbahn, G., Lohner, S., Kuhn, T., et al. (2020). Impact of intermittent energy restriction on anthropometric outcomes and intermediate disease markers in patients with overweight and obesity: Systematic review and meta-analyses. Critical Reviews in Food Science and Nutrition, 1–12.https://doi.org/10.1080/10408398.2020.1757616
Cioffi, I., Evangelista, A., Ponzo, V., Ciccone, G., Soldati, L., Santarpia, L., et al. (2018). Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. Journal of Translational Medicine, 16(1), 371. https://doi.org/10.1186/s12967-018-1748-4
Harris, L., Hamilton, S., Azevedo, L. B., Olajide, J., De Brun, C., Waller, G., et al. (2018). Intermittent fasting interventions for treatment of overweight and obesity in adults: A systematic review and meta-analysis. JBI Database of Systematic Reviews and Implementation Reports, 16(2), 507–547. https://doi.org/10.11124/JBISRIR-2016-003248
Farsijani, S., Payette, H., Morais, J. A., Shatenstein, B., Gaudreau, P., & Chevalier, S. (2017). Even mealtime distribution of protein intake is associated with greater muscle strength, but not with 3-y physical function decline, in free-living older adults: The Quebec longitudinal study on Nutrition as a Determinant of Successful Aging (NuAge study). American Journal of Clinical Nutrition, 106(1), 113–124. https://doi.org/10.3945/ajcn.116.146555
Halberg, N., Henriksen, M., Soderhamn, N., Stallknecht, B., Ploug, T., Schjerling, P., et al. (2005). Effect of intermittent fasting and refeeding on insulin action in healthy men. Journal of Applied Physiology (1985), 99(6), 2128–2136. https://doi.org/10.1152/japplphysiol.00683.2005
Soeters, M. R., Sauerwein, H. P., Groener, J. E., Aerts, J. M., Ackermans, M. T., Glatz, J. F., et al. (2007). Gender-related differences in the metabolic response to fasting. Journal of Clinical Endocrinology and Metabolism, 92(9), 3646–3652. https://doi.org/10.1210/jc.2007-0552
Miller, K. N., Burhans, M. S., Clark, J. P., Howell, P. R., Polewski, M. A., DeMuth, T. M., et al. (2017). Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell, 16(3), 497–507. https://doi.org/10.1111/acel.12575
Linford, N. J., Beyer, R. P., Gollahon, K., Krajcik, R. A., Malloy, V. L., Demas, V., et al. (2007). Transcriptional response to aging and caloric restriction in heart and adipose tissue. Aging Cell, 6(5), 673–688. https://doi.org/10.1111/j.1474-9726.2007.00319.x
Nisoli, E., Tonello, C., Cardile, A., Cozzi, V., Bracale, R., Tedesco, L., et al. (2005). Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science, 310(5746), 314–317. https://doi.org/10.1126/science.1117728
Okita, N., Hayashida, Y., Kojima, Y., Fukushima, M., Yuguchi, K., Mikami, K., et al. (2012). Differential responses of white adipose tissue and brown adipose tissue to caloric restriction in rats. Mechanisms of Ageing and Development, 133(5), 255–266. https://doi.org/10.1016/j.mad.2012.02.003
Giovannini, S., Carter, C. S., Leeuwenburgh, C., Flex, A., Biscetti, F., Morgan, D., et al. (2020). Effects of aging and life-long moderate calorie restriction on IL-15 signaling in the rat white adipose tissue. European Review for Medical and Pharmacological Sciences, 24(5), 2738–2749. https://doi.org/10.26355/eurrev_202003_20547
Kobayashi, M., Fujii, N., Narita, T., & Higami, Y. (2018). SREBP-1c-dependent metabolic remodeling of white adipose tissue by caloric restriction. International Journal of Molecular Sciences, 19(11). https://doi.org/10.3390/ijms19113335
Fujii, N., Uta, S., Kobayashi, M., Sato, T., Okita, N., & Higami, Y. (2019). Impact of aging and caloric restriction on fibroblast growth factor 21 signaling in rat white adipose tissue. Experimental Gerontology, 118, 55–64. https://doi.org/10.1016/j.exger.2019.01.001
Richman, E. L., Kenfield, S. A., Stampfer, M. J., Paciorek, A., Carroll, P. R., & Chan, J. M. (2011). Physical activity after diagnosis and risk of prostate cancer progression: Data from the cancer of the prostate strategic urologic research endeavor. Cancer Research, 71(11), 3889–3895. https://doi.org/10.1158/0008-5472.CAN-10-3932
Kenfield, S. A., Stampfer, M. J., Giovannucci, E., & Chan, J. M. (2011). Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. Journal of Clinical Oncology, 29(6), 726–732. https://doi.org/10.1200/JCO.2010.31.5226
Bonn, S. E., Sjolander, A., Lagerros, Y. T., Wiklund, F., Stattin, P., Holmberg, E., et al. (2015). Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiology, Biomarkers & Prevention, 24(1), 57–64. https://doi.org/10.1158/1055-9965.EPI-14-0707
Gunnell, A. S., Joyce, S., Tomlin, S., Taaffe, D. R., Cormie, P., Newton, R. U., et al. (2017). Physical activity and survival among long-term cancer survivor and non-cancer cohorts. Frontiers in Public Health, 5, 19. https://doi.org/10.3389/fpubh.2017.00019
McTiernan, A. (2008). Mechanisms linking physical activity with cancer. Nature Reviews Cancer, 8(3), 205–211. https://doi.org/10.1038/nrc2325
Winters-Stone, K. M., Wood, L. J., Stoyles, S., & Dieckmann, N. F. (2018). The effects of resistance exercise on biomarkers of breast cancer prognosis: A pooled analysis of three randomized trials. Cancer Epidemiology, Biomarkers & Prevention, 27(2), 146–153. https://doi.org/10.1158/1055-9965.Epi-17-0766
Dai, J. Y., Wang, B., Wang, X., Cheng, A., Kolb, S., Stanford, J. L., et al. (2019). Vigorous physical activity is associated with lower risk of metastatic-lethal progression in prostate cancer and hypomethylation in the CRACR2A gene. Cancer Epidemiology, Biomarkers & Prevention, 28(2), 258–264. https://doi.org/10.1158/1055-9965.EPI-18-0622
Kim, J. S., Galvao, D. A., Newton, R. U., Gray, E., & Taaffe, D. R. (2021). Exercise-induced myokines and their effect on prostate cancer. Nature Reviews. Urology, 18(9), 519–542. https://doi.org/10.1038/s41585-021-00476-y
Schmid, M., Martins, H. C., Schratt, G., Kropfl, J. M., & Spengler, C. M. (2021). MiRNA126 - RGS16 - CXCL12 cascade as a potential mechanism of acute exercise-induced precursor cell mobilization. Frontiers in Physiology, 12, 780666. https://doi.org/10.3389/fphys.2021.780666
Zaldivar, F., Eliakim, A., Radom-Aizik, S., Leu, S. Y., & Cooper, D. M. (2007). The effect of brief exercise on circulating CD34+ stem cells in early and late pubertal boys. Pediatric Research, 61(4), 491–495. https://doi.org/10.1203/pdr.0b013e3180332d36
Chang, E., Paterno, J., Duscher, D., Maan, Z. N., Chen, J. S., Januszyk, M., et al. (2015). Exercise induces stromal cell-derived factor-1alpha-mediated release of endothelial progenitor cells with increased vasculogenic function. Plastic and Reconstructive Surgery, 135(2), 340e–350e. https://doi.org/10.1097/PRS.0000000000000917
Niemiro, G. M., Parel, J., Beals, J., van Vliet, S., Paluska, S. A., Moore, D. R., et al. (2017). Kinetics of circulating progenitor cell mobilization during submaximal exercise. Journal of Applied Physiology (1985), 122(3), 675–682. https://doi.org/10.1152/japplphysiol.00936.2016
Niemiro, G. M., Allen, J. M., Mailing, L. J., Khan, N. A., Holscher, H. D., Woods, J. A., et al. (2018). Effects of endurance exercise training on inflammatory circulating progenitor cell content in lean and obese adults. Journal of Physiology, 596(14), 2811–2822. https://doi.org/10.1113/JP276023
Van Craenenbroeck, E. M., Beckers, P. J., Possemiers, N. M., Wuyts, K., Frederix, G., Hoymans, V. Y., et al. (2010). Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. European Heart Journal, 31(15), 1924–1934. https://doi.org/10.1093/eurheartj/ehq058
Van Craenenbroeck, E. M., Hoymans, V. Y., Beckers, P. J., Possemiers, N. M., Wuyts, K., Paelinck, B. P., et al. (2010). Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Research in Cardiology, 105(5), 665–676. https://doi.org/10.1007/s00395-010-0105-4
Ribeiro, F., Ribeiro, I. P., Goncalves, A. C., Alves, A. J., Melo, E., Fernandes, R., et al. (2017). Effects of resistance exercise on endothelial progenitor cell mobilization in women. Science and Reports, 7(1), 17880. https://doi.org/10.1038/s41598-017-18156-6
Ross, M. D., Malone, E. M., Simpson, R., Cranston, I., Ingram, L., Wright, G. P., et al. (2018). Lower resting and exercise-induced circulating angiogenic progenitors and angiogenic T cells in older men. American Journal of Physiology. Heart and Circulatory Physiology, 314(3), H392–H402. https://doi.org/10.1152/ajpheart.00592.2017
Wedell-Neergaard, A. S., Lang Lehrskov, L., Christensen, R. H., Legaard, G. E., Dorph, E., Larsen, M. K., et al. (2019). Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: A randomized controlled trial. Cell Metabolism, 29(4), 844–855 e843. https://doi.org/10.1016/j.cmet.2018.12.007
Khosravi, N., Stoner, L., Farajivafa, V., & Hanson, E. D. (2019). Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain, Behavior, and Immunity, 81, 92–104. https://doi.org/10.1016/j.bbi.2019.08.187
Rocha-Rodrigues, S., Matos, A., Afonso, J., Mendes-Ferreira, M., Abade, E., Teixeira, E., et al. (2021). Skeletal muscle-adipose tissue-tumor axis: Molecular mechanisms linking exercise training in prostate cancer. International Journal of Molecular Sciences, 22(9). https://doi.org/10.3390/ijms22094469
Idorn, M., & Thor Straten, P. (2018). Chemokine receptors and exercise to tackle the inadequacy of T cell homing to the tumor site. Cells, 7(8). https://doi.org/10.3390/cells7080108
Bourke, L., Smith, D., Steed, L., Hooper, R., Carter, A., Catto, J., et al. (2016). Exercise for men with prostate cancer: A systematic review and meta-analysis. European Urology, 69(4), 693–703. https://doi.org/10.1016/j.eururo.2015.10.047
Andersen, M. F., Midtgaard, J., & Bjerre, E. D. (2022). Do patients with prostate cancer benefit from exercise interventions? A systematic review and meta-analysis. International Journal of Environmental Research and Public Health, 19(2). https://doi.org/10.3390/ijerph19020972
Newton, R. U., Kenfield, S. A., Hart, N. H., Chan, J. M., Courneya, K. S., Catto, J., et al. (2018). Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): A multicentre, randomised, controlled phase III study protocol. British Medical Journal Open, 8(5), e022899. https://doi.org/10.1136/bmjopen-2018-022899
Kim, J. S., Taaffe, D. R., Galvao, D. A., Hart, N. H., Gray, E., Ryan, C. J., et al. (2022). Exercise in advanced prostate cancer elevates myokine levels and suppresses in-vitro cell growth. Prostate Cancer and Prostatic Diseases, 25(1), 86–92. https://doi.org/10.1038/s41391-022-00504-x
Ingram, D. K., Zhu, M., Mamczarz, J., Zou, S., Lane, M. A., Roth, G. S., et al. (2006). Calorie restriction mimetics: An emerging research field. Aging Cell, 5(2), 97–108. https://doi.org/10.1111/j.1474-9726.2006.00202.x
Salehi, B., Mishra, A. P., Nigam, M., Sener, B., Kilic, M., Sharifi-Rad, M., et al. (2018). Resveratrol: A double-edged sword in health benefits. Biomedicines, 6(3). https://doi.org/10.3390/biomedicines6030091
Lagouge, M., Argmann, C., Gerhart-Hines, Z., Meziane, H., Lerin, C., Daussin, F., et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell, 127(6), 1109–1122. https://doi.org/10.1016/j.cell.2006.11.013
Alberdi, G., Rodriguez, V. M., Miranda, J., Macarulla, M. T., Arias, N., Andres-Lacueva, C., et al. (2011). Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutrition & Metabolism (London), 8(1), 29. https://doi.org/10.1186/1743-7075-8-29
Cho, S. J., Jung, U. J., & Choi, M. S. (2012). Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. British Journal of Nutrition, 108(12), 2166–2175. https://doi.org/10.1017/S0007114512000347
Qiao, Y., Sun, J., Xia, S., Tang, X., Shi, Y., & Le, G. (2014). Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food & Function, 5(6), 1241–1249. https://doi.org/10.1039/c3fo60630a
Mendez-del Villar, M., Gonzalez-Ortiz, M., Martinez-Abundis, E., Perez-Rubio, K. G., & Lizarraga-Valdez, R. (2014). Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metabolic Syndrome and Related Disorders, 12(10), 497–501. https://doi.org/10.1089/met.2014.0082
Arzola-Paniagua, M. A., Garcia-Salgado Lopez, E. R., Calvo-Vargas, C. G., & Guevara-Cruz, M. (2016). Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity (Silver Spring), 24(7), 1454–1463. https://doi.org/10.1002/oby.21523
Faghihzadeh, F., Adibi, P., Rafiei, R., & Hekmatdoost, A. (2014). Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutrition Research, 34(10), 837–843. https://doi.org/10.1016/j.nutres.2014.09.005
Most, J., Timmers, S., Warnke, I., Jocken, J. W., van Boekschoten, M., de Groot, P., et al. (2016). Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. American Journal of Clinical Nutrition, 104(1), 215–227. https://doi.org/10.3945/ajcn.115.122937
Sheth, S., Jajoo, S., Kaur, T., Mukherjea, D., Sheehan, K., Rybak, L. P., et al. (2012). Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE, 7(12), e51655. https://doi.org/10.1371/journal.pone.0051655
Gill, C., Walsh, S. E., Morrissey, C., Fitzpatrick, J. M., & Watson, R. W. (2007). Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate, 67(15), 1641–1653. https://doi.org/10.1002/pros.20653
Tan, L., Wang, W., He, G., Kuick, R. D., Gossner, G., Kueck, A. S., et al. (2016). Resveratrol inhibits ovarian tumor growth in an in vivo mouse model. Cancer, 122(5), 722–729. https://doi.org/10.1002/cncr.29793
Li, W., Ma, J., Ma, Q., Li, B., Han, L., Liu, J., et al. (2013). Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-kappaB pathway. Current Medicinal Chemistry, 20(33), 4185–4194. https://doi.org/10.2174/09298673113209990251
Liang, Z. J., Wan, Y., Zhu, D. D., Wang, M. X., Jiang, H. M., Huang, D. L., et al. (2021). Resveratrol mediates the apoptosis of triple negative breast cancer cells by reducing POLD1 expression. Frontiers in Oncology, 11, 569295. https://doi.org/10.3389/fonc.2021.569295
Harper, C. E., Patel, B. B., Wang, J., Arabshahi, A., Eltoum, I. A., & Lamartiniere, C. A. (2007). Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis, 28(9), 1946–1953. https://doi.org/10.1093/carcin/bgm144
Wang, K., Chen, Z., Shi, J., Feng, Y., Yu, M., Sun, Y., et al. (2020). Resveratrol inhibits the tumor migration and invasion by upregulating TET1 and reducing TIMP2/3 methylation in prostate carcinoma cells. Prostate, 80(12), 977–985. https://doi.org/10.1002/pros.24029
Weisberg, S. P., Leibel, R., & Tortoriello, D. V. (2008). Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology, 149(7), 3549–3558. https://doi.org/10.1210/en.2008-0262
Shao, W., Yu, Z., Chiang, Y., Yang, Y., Chai, T., Foltz, W., et al. (2012). Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE, 7(1), e28784. https://doi.org/10.1371/journal.pone.0028784
Ejaz, A., Wu, D., Kwan, P., & Meydani, M. (2009). Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. Journal of Nutrition, 139(5), 919–925. https://doi.org/10.3945/jn.108.100966
Aggarwal, B. B., Kumar, A., & Bharti, A. C. (2003). Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Research, 23(1A), 363–398.
Vafadar, A., Shabaninejad, Z., Movahedpour, A., Fallahi, F., Taghavipour, M., Ghasemi, Y., et al. (2020). Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell & Bioscience, 10, 32. https://doi.org/10.1186/s13578-020-00397-0
Rivera, L., Moron, R., Sanchez, M., Zarzuelo, A., & Galisteo, M. (2008). Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring), 16(9), 2081–2087. https://doi.org/10.1038/oby.2008.315
Stewart, L. K., Soileau, J. L., Ribnicky, D., Wang, Z. Q., Raskin, I., Poulev, A., et al. (2008). Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism, 57(7 Suppl 1), S39-46. https://doi.org/10.1016/j.metabol.2008.03.003
Dong, J., Zhang, X., Zhang, L., Bian, H. X., Xu, N., Bao, B., et al. (2014). Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: A mechanism including AMPKalpha1/SIRT1. Journal of Lipid Research, 55(3), 363–374. https://doi.org/10.1194/jlr.M038786
He, Y., Cao, X., Guo, P., Li, X., Shang, H., Liu, J., et al. (2017). Quercetin induces autophagy via FOXO1-dependent pathways and autophagy suppression enhances quercetin-induced apoptosis in PASMCs in hypoxia. Free Radical Biology & Medicine, 103, 165–176. https://doi.org/10.1016/j.freeradbiomed.2016.12.016
Chondrogianni, N., Kapeta, S., Chinou, I., Vassilatou, K., Papassideri, I., & Gonos, E. S. (2010). Anti-ageing and rejuvenating effects of quercetin. Experimental Gerontology, 45(10), 763–771. https://doi.org/10.1016/j.exger.2010.07.001
Hoek-van den Hil, E. F., van Schothorst, E. M., van der Stelt, I., Swarts, H. J., Venema, D., Sailer, M., et al. (2014). Quercetin decreases high-fat diet induced body weight gain and accumulation of hepatic and circulating lipids in mice. Genes & Nutrition, 9(5), 418. https://doi.org/10.1007/s12263-014-0418-2
Mahini, H., Ainsworth, G., & Garelnabi, M. (2014). Exercise and quercetin intake reduces body weight in c57bl6 mice. Atherosclerosis, 235(2), e109–e110.
Pei, Y., Parks, J. S., & Kang, H. W. (2021). Quercetin alleviates high-fat diet-induced inflammation in brown adipose tissue. Journal of Functional Foods, 85.https://doi.org/10.1016/j.jff.2021.104614
Lee, J. S., Cha, Y. J., Lee, K. H., & Yim, J. E. (2016). Onion peel extract reduces the percentage of body fat in overweight and obese subjects: A 12-week, randomized, double-blind, placebo-controlled study. Nursing Research & Practice, 10(2), 175–181. https://doi.org/10.4162/nrp.2016.10.2.175
Pfeuffer, M., Auinger, A., Bley, U., Kraus-Stojanowic, I., Laue, C., Winkler, P., et al. (2013). Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutrition, Metabolism, and Cardiovascular Diseases, 23(5), 403–409. https://doi.org/10.1016/j.numecd.2011.08.010
Martin-Montalvo, A., Mercken, E. M., Mitchell, S. J., Palacios, H. H., Mote, P. L., Scheibye-Knudsen, M., et al. (2013). Metformin improves healthspan and lifespan in mice. Nature Communications, 4, 2192. https://doi.org/10.1038/ncomms3192
Anisimov, V. N., Berstein, L. M., Egormin, P. A., Piskunova, T. S., Popovich, I. G., Zabezhinski, M. A., et al. (2008). Metformin slows down aging and extends life span of female SHR mice. Cell Cycle, 7(17), 2769–2773. https://doi.org/10.4161/cc.7.17.6625
Saha, A., Blando, J., Tremmel, L., & DiGiovanni, J. (2015). Effect of metformin, rapamycin and their combination on growth and progression of prostate tumors in himyc mice. Cancer Prevention Research (Philadelphia, Pa.). https://doi.org/10.1158/1940-6207.CAPR-15-0014
Goodwin, P. J., & Stambolic, V. (2011). Obesity and insulin resistance in breast cancer–chemoprevention strategies with a focus on metformin. Breast, 20(Suppl 3), S31-35. https://doi.org/10.1016/S0960-9776(11)70291-0
Berstein, L. M. (2012). Metformin in obesity, cancer and aging: Addressing controversies. Aging (Albany NY), 4(5), 320–329. https://doi.org/10.18632/aging.100455
Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395. https://doi.org/10.1038/nature08221
Chang, G. R., Chiu, Y. S., Wu, Y. Y., Chen, W. Y., Liao, J. W., Chao, T. H., et al. (2009). Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. Journal of Pharmacological Sciences, 109(4), 496–503. https://doi.org/10.1254/jphs.08215FP
Checkley, L. A., Rho, O., Moore, T., Hursting, S., & DiGiovanni, J. (2011). Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prevention Research (Philadelphia, Pa.), 4(7), 1011–1020. https://doi.org/10.1158/1940-6207.CAPR-10-0375
Lee, D., Lee, J. H., Kim, B. H., Lee, S., Kim, D. W., & Kang, K. S. (2022). Phytochemical combination (p-synephrine, p-octopamine hydrochloride, and hispidulin) for improving obesity in obese mice induced by high-fat diet. Nutrients, 14(10). https://doi.org/10.3390/nu14102164
Lodi, A., Saha, A., Lu, X., Wang, B., Sentandreu, E., Collins, M., et al. (2017). Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. NPJ Precision Oncology, 1.https://doi.org/10.1038/s41698-017-0024-z
Daquinag, A. C., Dadbin, A., Snyder, B., Wang, X., Sahin, A., Ueno, N. T., et al. (2017). Non-glycanated decorin is a drug target on human adipose stromal cells. Molecular Therapy - Oncolytics, 6, 1–9.
Daquinag, A. C., Tseng, C., Zhang, Y., Amaya-Manzanares, F., Florez, F., Dadbin, A., et al. (2016). Targeted pro-apoptotic peptides depleting adipose stromal cells inhibit tumor growth. Molecular Therapy, 1, 34–40. https://doi.org/10.1038/mt.2015.155
Daquinag, A. C., Salameh, A., Zhang, Y., Tong, Q., & Kolonin, M. G. (2015). Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death & Diff., 22, 351–363. https://doi.org/10.1038/cdd.2014.148
Zaidi, N., Lupien, L., Kuemmerle, N. B., Kinlaw, W. B., Swinnen, J. V., & Smans, K. (2013). Lipogenesis and lipolysis: The pathways exploited by the cancer cells to acquire fatty acids. Progress in Lipid Research, 52(4), 585–589. https://doi.org/10.1016/j.plipres.2013.08.005
Ros, S., Santos, C. R., Moco, S., Baenke, F., Kelly, G., Howell, M., et al. (2012). Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discovery, 2(4), 328–343. https://doi.org/10.1158/2159-8290.CD-11-0234
Kuemmerle, N. B., Rysman, E., Lombardo, P. S., Flanagan, A. J., Lipe, B. C., Wells, W. A., et al. (2011). Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Molecular Cancer Therapeutics, 10(3), 427–436. https://doi.org/10.1158/1535-7163.MCT-10-0802
Domanska, U. M., Kruizinga, R. C., Nagengast, W. B., Timmer-Bosscha, H., Huls, G., de Vries, E. G., et al. (2013). A review on CXCR4/CXCL12 axis in oncology: No place to hide. European Journal of Cancer, 49(1), 219–230. https://doi.org/10.1016/j.ejca.2012.05.005
Zhao, H., Guo, L., Zhao, H., Zhao, J., Weng, H., & Zhao, B. (2015). CXCR4 over-expression and survival in cancer: A system review and meta-analysis. Oncotarget, 6(7), 5022–5040. https://doi.org/10.18632/oncotarget.3217
Darash-Yahana, M., Pikarsky, E., Abramovitch, R., Zeira, E., Pal, B., Karplus, R., et al. (2004). Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. The FASEB Journal, 18(11), 1240–1242. https://doi.org/10.1096/fj.03-0935fje
Cui, K., Zhao, W., Wang, C., Wang, A., Zhang, B., Zhou, W., et al. (2011). The CXCR4-CXCL12 pathway facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. [Research Support, Non-U.S. Gov’t]. Journal of Surgical Research, 171(1), 143–150. https://doi.org/10.1016/j.jss.2010.03.001
Teicher, B. A., & Fricker, S. P. (2010). CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clinical Cancer Research, 16(11), 2927–2931. https://doi.org/10.1158/1078-0432.CCR-09-2329
Scala, S. (2015). Molecular pathways: Targeting the CXCR4-CXCL12 axis–Untapped potential in the tumor microenvironment. Clinical Cancer Research, 21(19), 4278–4285. https://doi.org/10.1158/1078-0432.CCR-14-0914
Bleul, C. C., Fuhlbrigge, R. C., Casasnovas, J. M., Aiuti, A., & Springer, T. A. (1996). A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). Journal of Experimental Medicine, 184(3), 1101–1109. https://doi.org/10.1084/jem.184.3.1101
Hernandez, L., Magalhaes, M. A., Coniglio, S. J., Condeelis, J. S., & Segall, J. E. (2011). Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Research, 13(6), R128. https://doi.org/10.1186/bcr3074
Ahn, S., Saha, A., Kolonin, M. G., J. DiGiovanni, J. (2021). Signaling via both CXCR4 and CXCR7 in prostate cancer cells promotes tumor progression and underlies obesity-associated epithelial-mesenchymal transition. In revision
Funding
This work was supported in part by NIH grants R01 CA196259 and R01 CA228404 (to JD). Jill Hamilton-Reeves was supported by a Research Scholar Grant, RSG-17–050-01—NEC, from the American Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saha, A., Hamilton-Reeves, J. & DiGiovanni, J. White adipose tissue-derived factors and prostate cancer progression: mechanisms and targets for interventions. Cancer Metastasis Rev 41, 649–671 (2022). https://doi.org/10.1007/s10555-022-10056-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10056-0