Abstract
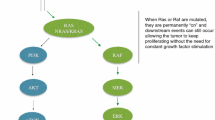
Colorectal cancer is a clinically and molecularly heterogeneous disease. Currently, extended RAS and BRAF mutation testing is obligatory in routine clinical practice before starting any treatment in the metastatic setting. Treatment decision making also includes assessment of the clinical condition of the patient, definition of the treatment goal, and consideration of the primary tumor site. Biological treatment is part of the first-line drug combination unless contraindicated. Mutational status is significantly associated with the outcome of patients and is strongly predictive for anti-EGFR-targeted therapy. The prognosis of RAS mutant CRC is clearly inferior to wild-type cases. RAS remains an elusive target, and specific treatment options are not yet available. Recently, promising results of a direct KRAS G12C inhibitor have been reported; however, further confirmation is needed. The biomarker landscape in mCRC is evolving; new promising markers are awaited with the chance of more precise targeted treatment.



Similar content being viewed by others
Abbreviations
- 5-FU:
-
5-Fluorouracil
- BRAF mut:
-
BRAF mutant
- CMS:
-
Consensus molecular subtypes
- CRC:
-
Colorectal cancer
- cfDNA:
-
Cell-free deoxyribonucleic acid
- ctDNA:
-
Circulating tumor deoxyribonucleic acid
- ddPCR:
-
Droplet digital polymerase chain reaction
- DNA:
-
Deoxyribonucleic acid
- EGFR:
-
Epidermal growth factor receptor
- ESMO:
-
European Society for Medical Oncology
- FFPE:
-
Formalin-fixed, paraffin-embedded
- FDA:
-
Food and Drug Administration
- FDP/TPI:
-
Trifluridine/tipiracil
- GDP:
-
Guanosine diphosphatase
- GTP:
-
Guanosine triphosphatase
- HRAS:
-
Harvey rat sarcoma viral oncogene homolog
- IVD:
-
In vitro diagnostic
- KRAS:
-
Kirsten rat sarcoma viral oncogene homolog
- MAPK:
-
Mitogen-activated protein kinase
- mCRC:
-
Metastatic colorectal cancer
- MSI:
-
Microsatellite instability
- MSS:
-
Microsatellite stable
- NCCN:
-
National Comprehensive Cancer Network
- NGS:
-
Next generation sequencing
- NRAS:
-
Neuroblastoma Ras viral oncogene homolog
- ORR:
-
Overall response rate
- OS:
-
Overall survival
- PCR:
-
Polymerase chain reaction
- PD-L1:
-
Programmed death-ligand 1
- PI3K:
-
Phosphoinositide 3-kinase
- PFS:
-
Progression-free survival
- PNA:
-
Peptide nucleic acid analog
- RAS mut:
-
RAS mutation
- RAS wt:
-
RAS wild type
- RR:
-
Response rate
- TML:
-
Through multiple lines
- VEGF:
-
Vascular endothelial growth factor
- VEGFR-2:
-
Vascular endothelial growth factor receptor 2
References
Siegel, R. L., Miller, K. D., Fedewa, S. A., Ahnen, D. J., Meester, R., Barzi, A., & Jemal, A. (2017). Colorectal cancer statistics, 2017. CA: a Cancer Journal for Clinicians, 67(3), 177–193. https://doi.org/10.3322/caac.21395.
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D. M., Forman, D., & Bray, F. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136(5), E359–E386. https://doi.org/10.1002/ijc.29210.
Riihimäki, M., Hemminki, A., Sundquist, J., & Hemminki, K. (2016). Patterns of metastasis in colon and rectal cancer. Scientific Reports, 6, 29765. https://doi.org/10.1038/srep2976.
Dienstmann, R., Connor, K., Byrne, A. T., & COLOSSUS Consortium. (2020). Precision therapy in RAS mutant colorectal cancer. Gastroenterology, 158(4), 806–811. https://doi.org/10.1053/j.gastro.2019.12.051.
Yoshino, T., Arnold, D., Taniguchi, H., Pentheroudakis, G., Yamazaki, K., Xu, R. H., Kim, T. W., Ismail, F., Tan, I. B., Yeh, K. H., Grothey, A., Zhang, S., Ahn, J. B., Mastura, M. Y., Chong, D., Chen, L. T., Kopetz, S., Eguchi-Nakajima, T., Ebi, H., Ohtsu, A., Cervantes, A., Muro, K., Tabernero, J., Minami, H., Ciardiello, F., & Douillard, J. Y. (2018). Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Annals of oncology : official journal of the European Society for Medical Oncology, 29(1), 44–70. https://doi.org/10.1093/annonc/mdx738.
Taieb, J., Jung, A., Sartore-Bianchi, A., Peeters, M., Seligmann, J., Zaanan, A., Burdon, P., Montagut, C., & Laurent-Puig, P. (2019). The evolving biomarker landscape for treatment selection in metastatic colorectal cancer. Drugs, 79(13), 1375–1394. https://doi.org/10.1007/s40265-019-01165-2.
Rauen, K. A. (2013). The RASopathies. Annual Review of Genomics and Human Genetics, 14, 355–369. https://doi.org/10.1146/annurev-genom-091212-153523.
Malumbres, M., & Barbacid, M. (2003). RAS oncogenes: The first 30 years. Nature Reviews. Cancer, 3(6), 459–465. https://doi.org/10.1038/nrc1097.
Jinesh, G. G., Sambandam, V., Vijayaraghavan, S., Balaji, K., & Mukherjee, S. (2018). Molecular genetics and cellular events of K-Ras-driven tumorigenesis. Oncogene, 37(7), 839–846. https://doi.org/10.1038/onc.2017.377.
Saeed, O., Lopez-Beltran, A., Fisher, K. W., Scarpelli, M., Montironi, R., Cimadamore, A., Massari, F., Santoni, M., & Cheng, L. (2019). RAS genes in colorectal carcinoma: Pathogenesis, testing guidelines and treatment implications. Journal of Clinical Pathology, 72(2), 135–139. https://doi.org/10.1136/jclinpath-2018-205471.
De Roock, W., Claes, B., Bernasconi, D., De Schutter, J., Biesmans, B., Fountzilas, G., Kalogeras, K. T., Kotoula, V., Papamichael, D., Laurent-Puig, P., Penault-Llorca, F., Rougier, P., Vincenzi, B., Santini, D., Tonini, G., Cappuzzo, F., Frattini, M., Molinari, F., Saletti, P., De Dosso, S., et al. (2010). Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. The Lancet. Oncology, 11(8), 753–762. https://doi.org/10.1016/S1470-2045(10)70130-3.
Cercek, A., Braghiroli, M. I., Chou, J. F., Hechtman, J. F., Kemeny, N., Saltz, L., Capanu, M., & Yaeger, R. (2017). Clinical features and outcomes of patients with colorectal cancers harboring NRAS mutations. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 23(16), 4753–4760. https://doi.org/10.1158/1078-0432.CCR-17-0400.
Wang, Y., Loree, J. M., Yu, C., Tschautscher, M., Briggler, A. M., et al. (2018). Distinct impacts of KRAS, NRAS and BRAF mutations on survival of patients with metastatic colorectal cancer. Journal of Clinical Oncology, 36(15_suppl), 3513. https://doi.org/10.1200/JCO.2018.36.15_suppl.3513.
Douillard, J. Y., Oliner, K. S., Siena, S., Tabernero, J., Burkes, R., Barugel, M., Humblet, Y., Bodoky, G., Cunningham, D., Jassem, J., Rivera, F., Kocákova, I., Ruff, P., Błasińska-Morawiec, M., Šmakal, M., Canon, J. L., Rother, M., Williams, R., Rong, A., Wiezorek, J., Sidhu, R., & Patterson, S. D. (2013). Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. The New England Journal of Medicine, 369(11), 1023–1034. https://doi.org/10.1056/NEJMoa1305275.
Sorich, M. J., Wiese, M. D., Rowland, A., Kichenadasse, G., McKinnon, R. A., & Karapetis, C. S. (2015). Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Annals of oncology : official journal of the European Society for Medical Oncology, 26(1), 13–21. https://doi.org/10.1093/annonc/mdu378.
Modest, D. P., Ricard, I., Heinemann, V., Hegewisch-Becker, S., Schmiegel, W., Porschen, R., Stintzing, S., Graeven, U., Arnold, D., von Weikersthal, L. F., Giessen-Jung, C., Stahler, A., Schmoll, H. J., Jung, A., Kirchner, T., Tannapfel, A., & Reinacher-Schick, A. (2016). Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Annals of oncology : official journal of the European Society for Medical Oncology, 27(9), 1746–1753. https://doi.org/10.1093/annonc/mdw261.
Camaj, P., Primo, S., Wang, Y., Heinemann, V., Zhao, Y., Laubender, R. P., Stintzing, S., Giessen-Jung, C., Jung, A., Gamba, S., Bruns, C. J., & Modest, D. P. (2015). KRAS exon 2 mutations influence activity of regorafenib in an SW48-based disease model of colorectal cancer. Future oncology (London, England), 11(13), 1919–1929. https://doi.org/10.2217/fon.15.97.
Fiala, O., Buchler, T., Mohelnikova-Duchonova, B., Melichar, B., Matejka, V. M., Holubec, L., Kulhankova, J., Bortlicek, Z., Bartouskova, M., Liska, V., Topolcan, O., Sedivcova, M., & Finek, J. (2016). G12V and G12A KRAS mutations are associated with poor outcome in patients with metastatic colorectal cancer treated with bevacizumab. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 37(5), 6823–6830. https://doi.org/10.1007/s13277-015-4523-7.
Tol, J., Nagtegaal, I. D., & Punt, C. J. (2009). BRAF mutation in metastatic colorectal cancer. The New England Journal of Medicine, 361(1), 98–99. https://doi.org/10.1056/NEJMc0904160.
Venderbosch, S., Nagtegaal, I. D., Maughan, T. S., Smith, C. G., Cheadle, J. P., Fisher, D., Kaplan, R., Quirke, P., Seymour, M. T., Richman, S. D., Meijer, G. A., Ylstra, B., Heideman, D. A., de Haan, A. F., Punt, C. J., & Koopman, M. (2014). Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 20(20), 5322–5330. https://doi.org/10.1158/1078-0432.CCR-14-0332.
Michaloglou, C., Vredeveld, L. C., Mooi, W. J., & Peeper, D. S. (2008). BRAF(E600) in benign and malignant human tumours. Oncogene, 27(7), 877–895. https://doi.org/10.1038/sj.onc.1210704.
Ogino, S., Brahmandam, M., Cantor, M., Namgyal, C., Kawasaki, T., Kirkner, G., Meyerhardt, J. A., Loda, M., & Fuchs, C. S. (2006). Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 19(1), 59–68. https://doi.org/10.1038/modpathol.3800482.
Jones, J. C., Kipp, B., Leal, A. D., Voss, J. S., Hubbard, J. M., McWilliams, R. R., et al. (2016). Commonality and clinical, pathological, and prognostic characteristics of nonV600E BRAF mutations (BRAFMut) in metastatic colorectal cancers (mCRC) compared to V600 BRAFMut CRCs. Journal of Clinical Oncology, 34(15_suppl), 3529. https://doi.org/10.1200/JCO.2016.34.15_suppl.3529.
Nollau, P., & Wagener, C. (1997). Methods for detection of point mutations: Performance and quality assessment. IFCC Scientific Division, Committee on Molecular Biology Techniques. Clinical Chemistry, 43(7), 1114–1128.
Castle, G., & Blaney, R. (2010). European Union regulation of in vitro diagnostic medical devices. In S. D. Danzis & E. J. Flannery (Eds.), In vitro diagnostics: The complete regulatory guide (pp. 227–252). Washington D.C.: Food and Drug Law Institute.
European Society of Pathology. General report. ESP KRAS external quality assessment scheme 2011. http://kras.eqascheme.org. Published March 9, 2012.
Frayling, I. M. (2002). Methods of molecular analysis: Mutation detection in solid tumours. Molecular pathology : MP, 55(2), 73–79. https://doi.org/10.1136/mp.55.2.73.
Amado, R. G., Wolf, M., Peeters, M., Van Cutsem, E., Siena, S., Freeman, D. J., Juan, T., Sikorski, R., Suggs, S., Radinsky, R., Patterson, S. D., & Chang, D. D. (2008). Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 26(10), 1626–1634. https://doi.org/10.1200/JCO.2007.14.7116.
Tol, J., Koopman, M., Cats, A., Rodenburg, C. J., Creemers, G. J., Schrama, J. G., Erdkamp, F. L., Vos, A. H., van Groeningen, C. J., Sinnige, H. A., Richel, D. J., Voest, E. E., Dijkstra, J. R., Vink-Börger, M. E., Antonini, N. F., Mol, L., van Krieken, J. H., Dalesio, O., & Punt, C. J. (2009). Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. The New England Journal of Medicine, 360(6), 563–572. https://doi.org/10.1056/NEJMoa0808268.
Harlé, A., Filhine-Tresarrieu, P., Husson, M., Boidot, R., Rouyer, M., Dubois, C., Leroux, A., & Merlin, J. L. (2016). Rare RAS mutations in metastatic colorectal cancer detected during routine RAS genotyping using next generation sequencing. Targeted Oncology, 11(3), 363–370. https://doi.org/10.1007/s11523-015-0404-7.
Beau-Faller, M., Legrain, M., Voegeli, A. C., Guérin, E., Lavaux, T., Ruppert, A. M., Neuville, A., Massard, G., Wihlm, J. M., Quoix, E., Oudet, P., & Gaub, M. P. (2009). Detection of K-Ras mutations in tumour samples of patients with non-small cell lung cancer using PNA-mediated PCR clamping. British Journal of Cancer, 100(6), 985–992. https://doi.org/10.1038/sj.bjc.6604925.
Lee, S., Brophy, V. H., Cao, J., Velez, M., Hoeppner, C., Soviero, S., & Lawrence, H. J. (2012). Analytical performance of a PCR assay for the detection of KRAS mutations (codons 12/13 and 61) in formalin-fixed paraffin-embedded tissue samples of colorectal carcinoma. Virchows Archiv : an international journal of pathology, 460(2), 141–149. https://doi.org/10.1007/s00428-011-1180-0.
Gonzalez de Castro, D., Angulo, B., Gomez, B., Mair, D., Martinez, R., Suarez-Gauthier, A., Shieh, F., Velez, M., Brophy, V. H., Lawrence, H. J., & Lopez-Rios, F. (2012). A comparison of three methods for detecting KRAS mutations in formalin-fixed colorectal cancer specimens. British Journal of Cancer, 107(2), 345–351. https://doi.org/10.1038/bjc.2012.259.
Franklin, W. A., Haney, J., Sugita, M., Bemis, L., Jimeno, A., & Messersmith, W. A. (2010). KRAS mutation: Comparison of testing methods and tissue sampling techniques in colon cancer. The Journal of molecular diagnostics : JMD, 12(1), 43–50. https://doi.org/10.2353/jmoldx.2010.080131.
Kriegshäuser, G., Auner, V., Schuster, E., Holzer, B., Oberkanins, C., Horvat, R., Speiser, P., & Zeillinger, R. (2011). KRAS mutation analysis in genomic DNA isolated from formalin-fixed paraffin-embedded ovarian tissue: Evaluation of a strip-based reverse-hybridisation assay. Journal of Clinical Pathology, 64(3), 252–256. https://doi.org/10.1136/jcp.2010.081414.
Gao J, Wu H, Wang L, et al. Validation of targeted next-generation sequencing for RAS mutation detection in FFPE colorectal cancer tissues: Comparison with Sanger sequencing and ARMS-Scorpion real-time PCR. BMJ Open. 2016;6(1):e009532. Published 2016 Jan 8. doi:https://doi.org/10.1136/bmjopen-2015-009532
Albanese, I., Scibetta, A. G., Migliavacca, M., Russo, A., Bazan, V., Tomasino, R. M., Colomba, P., Tagliavia, M., & la Farina, M. (2004). Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochemical and Biophysical Research Communications, 325(3), 784–791. https://doi.org/10.1016/j.bbrc.2004.10.111.
Losi, L., Baisse, B., Bouzourene, H., & Benhattar, J. (2005). Evolution of intratumoral genetic heterogeneity during colorectal cancer progression. Carcinogenesis., 26(5), 916–922. https://doi.org/10.1093/carcin/bgi044.
Gerlinger, M., Rowan, A. J., Horswell, S., Math, M., Larkin, J., Endesfelder, D., Gronroos, E., Martinez, P., Matthews, N., Stewart, A., Tarpey, P., Varela, I., Phillimore, B., Begum, S., McDonald, N. Q., Butler, A., Jones, D., Raine, K., Latimer, C., Santos, C. R., et al. (2012). Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England Journal of Medicine, 366(10), 883–892. https://doi.org/10.1056/NEJMoa1113205.
Turner, N. C., & Reis-Filho, J. S. (2012). Genetic heterogeneity and cancer drug resistance. The Lancet. Oncology, 13(4), e178–e185. https://doi.org/10.1016/S1470-2045(11)70335-7.
Stroun, M., Anker, P., Lyautey, J., Lederrey, C., & Maurice, P. A. (1987). Isolation and characterization of DNA from the plasma of cancer patients. European Journal of Cancer & Clinical Oncology, 23(6), 707–712. https://doi.org/10.1016/0277-5379(87)90266-5.
Diehl, F., Schmidt, K., Choti, M. A., Romans, K., Goodman, S., Li, M., Thornton, K., Agrawal, N., Sokoll, L., Szabo, S. A., Kinzler, K. W., Vogelstein, B., & Diaz Jr., L. A. (2008). Circulating mutant DNA to assess tumor dynamics. Nature Medicine, 14(9), 985–990. https://doi.org/10.1038/nm.1789.
Diaz Jr., L. A., & Bardelli, A. (2014). Liquid biopsies: Genotyping circulating tumor DNA. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 32(6), 579–586. https://doi.org/10.1200/JCO.2012.45.2011.
Banks, KC., Mortimer, SAW., Zill, OA., Lanman, RB., Eltoukhy, H., Talasaz, AA. (2015). Genomic profiling of over 5,000 consecutive cancer patients with a CLIA-certified cell-free DNA NGS test: Analytic and clinical validity and utility. In: Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; 2015 Nov 5-9; Boston, MA. Philadelphia (PA): AACR; Mol Cancer Ther 14 (12 Suppl 2): Abstract nr B140. DOI: https://doi.org/10.1158/1535-7163.TARG-15-B140.
Forshew, T., Murtaza, M., Parkinson, C., Gale, D., Tsui, D. W., Kaper, F., Dawson, S. J., Piskorz, A. M., Jimenez-Linan, M., Bentley, D., Hadfield, J., May, A. P., Caldas, C., Brenton, J. D., & Rosenfeld, N. (2012). Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Science Translational Medicine, 4(136), 136ra68. https://doi.org/10.1126/scitranslmed.3003726.
Newman, A. M., Bratman, S. V., To, J, Wynne, J. F., Eclov, N. C., Modlin, L. A., Liu, C. L., Neal, J. W., Wakelee, H. A., Merritt, R. E., Shrager, J. B., Loo Jr., B. W., Alizadeh, A. A., & Diehn, M. (2014). An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nature Medicine, 20(5), 548–554. https://doi.org/10.1038/nm.3519.
Lanman, R. B., Mortimer, S. A., Zill, O. A., Sebisanovic, D., Lopez, R., Blau, S., Collisson, E. A., Divers, S. G., Hoon, D. S., Kopetz, E. S., Lee, J., Nikolinakos, P. G., Baca, A. M., Kermani, B. G., Eltoukhy, H., & Talasaz, A. (2015). Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One, 10(10), e0140712. https://doi.org/10.1371/journal.pone.0140712.
Shu, Y., Wu, X., Tong, X., Wang, X., Chang, Z., Mao, Y., Chen, X., Sun, J., Wang, Z., Hong, Z., Zhu, L., Zhu, C., Chen, J., Liang, Y., Shao, H., & Shao, Y. W. (2017). Circulating tumor DNA mutation profiling by targeted next generation sequencing provides guidance for personalized treatments in multiple cancer types. Scientific Reports, 7(1), 583. https://doi.org/10.1038/s41598-017-00520-1.
Schwaederle, M., Husain, H., Fanta, P. T., Piccioni, D. E., Kesari, S., Schwab, R. B., Patel, S. P., Harismendy, O., Ikeda, M., Parker, B. A., & Kurzrock, R. (2016). Use of liquid biopsies in clinical oncology: Pilot experience in 168 patients. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 22(22), 5497–5505. https://doi.org/10.1158/1078-0432.CCR-16-0318.
Schwaederle, M., Husain, H., Fanta, P. T., Piccioni, D. E., Kesari, S., Schwab, R. B., Banks, K. C., Lanman, R. B., Talasaz, A., Parker, B. A., & Kurzrock, R. (2016). Detection rate of actionable mutations in diverse cancers using a biopsy-free (blood) circulating tumor cell DNA assay. Oncotarget, 7(9), 9707–9717. https://doi.org/10.18632/oncotarget.7110.
Demuth, C., Spindler, K. G., Johansen, J. S., Pallisgaard, N., Nielsen, D., Hogdall, E., Vittrup, B., & Sorensen, B. S. (2018). Measuring KRAS mutations in circulating tumor DNA by droplet digital PCR and next-generation sequencing. Translational Oncology, 11(5), 1220–1224. https://doi.org/10.1016/j.tranon.2018.07.013.
Garcia, J., Forestier, J., Dusserre, E., Wozny, A. S., Geiguer, F., Merle, P., Tissot, C., Ferraro-Peyret, C., Jones, F. S., Edelstein, D. L., Cheynet, V., Bardel, C., Vilchez, G., Xu, Z., Bringuier, P. P., Barritault, M., Brengle-Pesce, K., Guillet, M., Chauvenet, M., Manship, B., Brevet, M., Rodriguez-Lafrasse, C., Hervieu, V., Couraud, S., Walter, T., & Payen, L. (2018). Cross-platform comparison for the detection of RAS mutations in cfDNA (ddPCR Biorad detection assay, BEAMing assay, and NGS strategy). Oncotarget, 9(30), 21122–21131. https://doi.org/10.18632/oncotarget.24950.
Kim, M. J., Lee, H. S., Kim, J. H., Kim, Y. J., Kwon, J. H., Lee, J. O., Bang, S. M., Park, K. U., Kim, D. W., Kang, S. B., Kim, J. S., Lee, J. S., & Lee, K. W. (2012). Different metastatic pattern according to the KRAS mutational status and site-specific discordance of KRAS status in patients with colorectal cancer. BMC Cancer, 12, 347. https://doi.org/10.1186/1471-2407-12-347.
Choi, I. S., Kato, S., Fanta, P. T., Leichman, L., Okamura, R., Raymond, V. M., Lanman, R. B., Lippman, S. M., & Kurzrock, R. (2019). Genomic profiling of blood-derived circulating tumor DNA from patients with colorectal cancer: Implications for response and resistance to targeted therapeutics. Molecular Cancer Therapeutics, 18(10), 1852–1862. https://doi.org/10.1158/1535-7163.MCT-18-0965.
Thierry, A. R., El Messaoudi, S., Mollevi, C., Raoul, J. L., Guimbaud, R., Pezet, D., Artru, P., Assenat, E., Borg, C., Mathonnet, M., De La Fouchardière, C., Bouché, O., Gavoille, C., Fiess, C., Auzemery, B., Meddeb, R., Lopez-Crapez, E., Sanchez, C., Pastor, B., & Ychou, M. (2017). Clinical utility of circulating DNA analysis for rapid detection of actionable mutations to select metastatic colorectal patients for anti-EGFR treatment. Annals of oncology : official journal of the European Society for Medical Oncology, 28(9), 2149–2159. https://doi.org/10.1093/annonc/mdx330.
Normanno, N., Cervantes, A., Ciardiello, F., De Luca, A., & Pinto, C. (2018). The liquid biopsy in the management of colorectal cancer patients: Current applications and future scenarios. Cancer Treatment Reviews, 70, 1–8. https://doi.org/10.1016/j.ctrv.2018.07.007.
Blons, H., Emile, J. F., Le Malicot, K., Julié, C., Zaanan, A., Tabernero, J., Mini, E., Folprecht, G., Van Laethem, J. L., Thaler, J., Bridgewater, J., Nørgård-Petersen, L., Van Cutsem, E., Lepage, C., Zawadi, M. A., Salazar, R., Laurent-Puig, P., Taieb, J., & PETACC-8 Study Investigators. (2014). Prognostic value of KRAS mutations in stage III colon cancer: Post hoc analysis of the PETACC8 phase III trial dataset. Annals of oncology : official journal of the European Society for Medical Oncology, 25(12), 2378–2385. https://doi.org/10.1093/annonc/mdu464.
Sinicrope, F. A., Mahoney, M. R., Yoon, H. H., Smyrk, T. C., Thibodeau, S. N., Goldberg, R. M., Nelson, G. D., Sargent, D. J., Alberts, S. R., & Alliance for Clinical Trials in Oncology. (2015). Analysis of molecular markers by anatomic tumor site in stage III colon carcinomas from adjuvant chemotherapy trial NCCTG N0147 (Alliance). Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 21(23), 5294–5304. https://doi.org/10.1158/1078-0432.CCR-15-0527.
Gavin, P. G., Colangelo, L. H., Fumagalli, D., Tanaka, N., Remillard, M. Y., Yothers, G., Kim, C., Taniyama, Y., Kim, S. I., Choi, H. J., Blackmon, N. L., Lipchik, C., Petrelli, N. J., O'Connell, M. J., Wolmark, N., Paik, S., & Pogue-Geile, K. L. (2012). Mutation profiling and microsatellite instability in stage II and III colon cancer: An assessment of their prognostic and oxaliplatin predictive value. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 18(23), 6531–6541. https://doi.org/10.1158/1078-0432.CCR-12-0605.
Kyu, H. H., Bachman, V. F., Alexander, L. T., Mumford, J. E., Afshin, A., Estep, K., Veerman, J. L., Delwiche, K., Iannarone, M. L., Moyer, M. L., Cercy, K., Vos, T., Murray, C. J., & Forouzanfar, M. H. (2016). Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ (Clinical research ed.), 354, i3857. https://doi.org/10.1136/bmj.i3857.
Allegra, C. J., Jessup, J. M., Somerfield, M. R., Hamilton, S. R., Hammond, E. H., Hayes, D. F., McAllister, P. K., Morton, R. F., & Schilsky, R. L. (2009). American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 27(12), 2091–2096. https://doi.org/10.1200/JCO.2009.21.9170.
Allegra, C. J., Rumble, R. B., Hamilton, S. R., Mangu, P. B., Roach, N., Hantel, A., & Schilsky, R. L. (2016). Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 34(2), 179–185. https://doi.org/10.1200/JCO.2015.63.9674.
Van Cutsem, E., Lenz, H. J., Köhne, C. H., Heinemann, V., Tejpar, S., Melezínek, I., Beier, F., Stroh, C., Rougier, P., van Krieken, J. H., & Ciardiello, F. (2015). Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 33(7), 692–700. https://doi.org/10.1200/JCO.2014.59.4812.
Saltz, L. B., Clarke, S., Díaz-Rubio, E., Scheithauer, W., Figer, A., Wong, R., Koski, S., Lichinitser, M., Yang, T. S., Rivera, F., Couture, F., Sirzén, F., & Cassidy, J. (2008). Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 26(12), 2013–2019. https://doi.org/10.1200/JCO.2007.14.9930.
Passardi, A., Nanni, O., Tassinari, D., Turci, D., Cavanna, L., Fontana, A., Ruscelli, S., Mucciarini, C., Lorusso, V., Ragazzini, A., Frassineti, G. L., & Amadori, D. (2015). Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: Final results for first-line treatment from the ITACa randomized clinical trial. Annals of oncology : official journal of the European Society for Medical Oncology, 26(6), 1201–1207. https://doi.org/10.1093/annonc/mdv130.
Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W., Berlin, J., Baron, A., Griffing, S., Holmgren, E., Ferrara, N., Fyfe, G., Rogers, B., Ross, R., & Kabbinavar, F. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England Journal of Medicine, 350(23), 2335–2342. https://doi.org/10.1056/NEJMoa032691.
Ocvirk, J., Brodowicz, T., Wrba, F., Ciuleanu, T. E., Kurteva, G., Beslija, S., Koza, I., Pápai, Z., Messinger, D., Yilmaz, U., Faluhelyi, Z., Yalcin, S., Papamichael, D., Wenczl, M., Mrsic-Krmpotic, Z., Shacham-Shmueli, E., Vrbanec, D., Esser, R., Scheithauer, W., & Zielinski, C. C. (2010). Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World Journal of Gastroenterology, 16(25), 3133–3143. https://doi.org/10.3748/wjg.v16.i25.3133.
Parikh, A. R., Lee, F. C., Yau, L., Koh, H., Knost, J., Mitchell, E. P., Bosanac, I., Choong, N., Scappaticci, F., Mancao, C., & Lenz, H. J. (2019). MAVERICC, a randomized, biomarker-stratified, phase II study of mFOLFOX6-Bevacizumab versus FOLFIRI-Bevacizumab as first-line chemotherapy in metastatic colorectal cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 25(10), 2988–2995. https://doi.org/10.1158/1078-0432.CCR-18-1221.
Venook, A. P., Niedzwiecki, D., Lenz, H. J., Innocenti, F., Fruth, B., Meyerhardt, J. A., Schrag, D., Greene, C., O'Neil, B. H., Atkins, J. N., Berry, S., Polite, B. N., O'Reilly, E. M., Goldberg, R. M., Hochster, H. S., Schilsky, R. L., Bertagnolli, M. M., El-Khoueiry, A. B., Watson, P., Benson 3rd, A. B., et al. (2017). Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA, 317(23), 2392–2401. https://doi.org/10.1001/jama.2017.7105.
Stintzing, S., Modest, D. P., Rossius, L., Lerch, M. M., von Weikersthal, L. F., Decker, T., Kiani, A., Vehling-Kaiser, U., Al-Batran, S. E., Heintges, T., Lerchenmüller, C., Kahl, C., Seipelt, G., Kullmann, F., Stauch, M., Scheithauer, W., Held, S., Giessen-Jung, C., Moehler, M., Jagenburg, A., et al. (2016). FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. The Lancet. Oncology, 17(10), 1426–1434. https://doi.org/10.1016/S1470-2045(16)30269-8.
Rivera, F., Karthaus, M., Hecht, J. R., Sevilla, I., Forget, F., Fasola, G., Canon, J. L., Guan, X., Demonty, G., & Schwartzberg, L. S. (2017). Final analysis of the randomised PEAK trial: Overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. International Journal of Colorectal Disease, 32(8), 1179–1190. https://doi.org/10.1007/s00384-017-2800-1.
Khattak, M. A., Martin, H., Davidson, A., & Phillips, M. (2015). Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: A meta-analysis of randomized clinical trials. Clinical Colorectal Cancer, 14(2), 81–90. https://doi.org/10.1016/j.clcc.2014.12.011.
Heinemann, V., Rivera, F., O'Neil, B. H., Stintzing, S., Koukakis, R., Terwey, J. H., & Douillard, J. Y. (2016). A study-level meta-analysis of efficacy data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in patients with RAS wild-type metastatic colorectal cancer. European journal of cancer (Oxford, England : 1990), 67, 11–20. https://doi.org/10.1016/j.ejca.2016.07.019.
Loupakis, F., Yang, D., Yau, L., Feng, S., Cremolini, C., Zhang, W., Maus, M. K., Antoniotti, C., Langer, C., Scherer, S. J., Müller, T., Hurwitz, H. I., Saltz, L., Falcone, A., & Lenz, H. J. (2015). Primary tumor location as a prognostic factor in metastatic colorectal cancer. Journal of the National Cancer Institute, 107(3), dju427. https://doi.org/10.1093/jnci/dju427.
Arnold, D., Lueza, B., Douillard, J. Y., Peeters, M., Lenz, H. J., Venook, A., Heinemann, V., Van Cutsem, E., Pignon, J. P., Tabernero, J., Cervantes, A., & Ciardiello, F. (2017). Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Annals of oncology : official journal of the European Society for Medical Oncology, 28(8), 1713–1729. https://doi.org/10.1093/annonc/mdx175.
Ciardiello, F., Lenz, H. J., Kohne, C. H., et al. (2014). Effect of KRAS and NRAS mutational status on first-line treatment with FOLFIRI plus cetuximab in patients with metastatic colorectal cancer (mCRC): New results from the CRYSTAL trial. Journal of Clinical Oncology, 32(3_suppl). https://doi.org/10.1200/jco.2014.32.3_suppl.lba443.
Kubicka, S., Greil, R., André, T., Bennouna, J., Sastre, J., Van Cutsem, E., von Moos, R., Osterlund, P., Reyes-Rivera, I., Müller, T., Makrutzki, M., Arnold, D., & ML18147 study investigators including AIO, GERCOR, FFCD, UNICANCER GI, TTD, BGDO, GEMCAD, and AGMT groups. (2013). Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Annals of oncology : official journal of the European Society for Medical Oncology, 24(9), 2342–2349. https://doi.org/10.1093/annonc/mdt231.
Xu, J., Liu, T., Tang, W., Chang, W., Feng, Q., et al. (2019). Bevacizumab plus chemotherapy versus chemotherapy alone as firstline treatment for patients with RAS mutant unresectable colorectal liver-limited metastases: A single center randomized control trial. Annals of Oncology, 30(suppl_5), 851–934. https://doi.org/10.1093/annonc/mdz394.020.
Cremolini, C., Loupakis, F., Antoniotti, C., Lupi, C., Sensi, E., Lonardi, S., Mezi, S., Tomasello, G., Ronzoni, M., Zaniboni, A., Tonini, G., Carlomagno, C., Allegrini, G., Chiara, S., D'Amico, M., Granetto, C., Cazzaniga, M., Boni, L., Fontanini, G., & Falcone, A. (2015). FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. The Lancet. Oncology, 16(13), 1306–1315. https://doi.org/10.1016/S1470-2045(15)00122-9.
Bendell, J. C., Kim, T. W., Goh, B. C., Wallin, J., Oh, D.-Y., Han, S.-W., Lee, C. B., Hellmann, M. D., Desai, J., Lewin, J. H., Solomon, B. J., Chow, L. Q. M., Miller, W. H., Gainor, J. F., Flaherty, K., Infante, J. R., Das-Thakur, M., Foster, P., Cha, E., & Bang, Y. J. (2016). Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). Journal of Clinical Oncology, 34, 3502. https://doi.org/10.1200/JCO.2016.34.15_suppl.3502.
McGregor, M., & Price, T. J. (2019). IMblaze 370: Lessons learned and future strategies in colorectal cancer treatment. Annals of translational medicine, 7(21), 602. https://doi.org/10.21037/atm.2019.08.119.
Govindan, R., Fakih, M. G., Price, T. J., Falchook, G. S., Desai, J., Kuo, J. C., et al. (2019). Phase 1 study of AMG 510, a novel molecule targeting KRAS G12C mutant solid tumors. Annals of Oncology, 30(suppl_5), 159–193. https://doi.org/10.1093/annonc/mdz244.008.
Passot, G., Denbo, J. W., Yamashita, S., Kopetz, S. E., Chun, Y. S., Maru, D., Overman, M. J., Brudvik, K. W., Conrad, C., Aloia, T. A., & Vauthey, J. N. (2017). Is hepatectomy justified for patients with RAS mutant colorectal liver metastases? An analysis of 524 patients undergoing curative liver resection. Surgery, 161(2), 332–340. https://doi.org/10.1016/j.surg.2016.07.032.
Vauthey, J. N., Zimmitti, G., Kopetz, S. E., Shindoh, J., Chen, S. S., Andreou, A., Curley, S. A., Aloia, T. A., & Maru, D. M. (2013). RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Annals of Surgery, 258(4), 619–627. https://doi.org/10.1097/SLA.0b013e3182a5025a.
Schweiger, T., Hegedüs, B., Nikolowsky, C., Hegedüs, Z., Szirtes, I., Mair, R., Birner, P., Döme, B., Lang, G., Klepetko, W., Ankersmit, H. J., & Hoetzenecker, K. (2014). EGFR, BRAF and KRAS status in patients undergoing pulmonary metastasectomy from primary colorectal carcinoma: A prospective follow-up study. Annals of Surgical Oncology, 21(3), 946–954. https://doi.org/10.1245/s10434-013-3386-7.
Bennouna, J., Sastre, J., Arnold, D., Österlund, P., Greil, R., Van Cutsem, E., von Moos, R., Viéitez, J. M., Bouché, O., Borg, C., Steffens, C. C., Alonso-Orduña, V., Schlichting, C., Reyes-Rivera, I., Bendahmane, B., André, T., Kubicka, S., & ML18147 Study Investigators. (2013). Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. The Lancet. Oncology, 14(1), 29–37. https://doi.org/10.1016/S1470-2045(12)70477-1.
Tabernero, J., Van Cutsem, E., Lakomý, R., Prausová, J., Ruff, P., van Hazel, G. A., Moiseyenko, V. M., Ferry, D. R., McKendrick, J. J., Soussan-Lazard, K., Chevalier, S., & Allegra, C. J. (2014). Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: Prespecified subgroup analyses from the VELOUR trial. European journal of cancer (Oxford, England : 1990), 50(2), 320–331. https://doi.org/10.1016/j.ejca.2013.09.013.
Van Cutsem, E., Mayer, R. J., Laurent, S., Winkler, R., Grávalos, C., Benavides, M., Longo-Munoz, F., Portales, F., Ciardiello, F., Siena, S., Yamaguchi, K., Muro, K., Denda, T., Tsuji, Y., Makris, L., Loehrer, P., Lenz, H. J., Ohtsu, A., & RECOURSE Study Group. (2018). The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. European journal of cancer (Oxford, England : 1990), 90, 63–72. https://doi.org/10.1016/j.ejca.2017.10.009.
Grothey, A., Van Cutsem, E., Sobrero, A., Siena, S., Falcone, A., Ychou, M., Humblet, Y., Bouché, O., Mineur, L., Barone, C., Adenis, A., Tabernero, J., Yoshino, T., Lenz, H. J., Goldberg, R. M., Sargent, D. J., Cihon, F., Cupit, L., Wagner, A., Laurent, D., et al. (2013). Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (London, England), 381(9863), 303–312. https://doi.org/10.1016/S0140-6736(12)61900-X.
Misale, S., Yaeger, R., Hobor, S., Scala, E., Janakiraman, M., Liska, D., Valtorta, E., Schiavo, R., Buscarino, M., Siravegna, G., Bencardino, K., Cercek, A., Chen, C. T., Veronese, S., Zanon, C., Sartore-Bianchi, A., Gambacorta, M., Gallicchio, M., Vakiani, E., Boscaro, V., Medico, E., Weiser, M., Siena, S., di Nicolantonio, F., Solit, D., & Bardelli, A. (2012). Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature, 486(7404), 532–536. https://doi.org/10.1038/nature11156.
Goldberg, R. M., Montagut, C., Wainberg, Z. A., Ronga, P., Audhuy, F., Taieb, J., Stintzing, S., Siena, S., & Santini, D. (2018). Optimising the use of cetuximab in the continuum of care for patients with metastatic colorectal cancer. ESMO open, 3(4), e000353. https://doi.org/10.1136/esmoopen-2018-000353.
Cremolini, C., Rossini, D., Dell'Aquila, E., Lonardi, S., Conca, E., Del Re, M., Busico, A., Pietrantonio, F., Danesi, R., Aprile, G., Tamburini, E., Barone, C., Masi, G., Pantano, F., Pucci, F., Corsi, D. C., Pella, N., Bergamo, F., Rofi, E., Barbara, C., et al. (2019). Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: A phase 2 single-arm clinical trial. JAMA Oncology, 5(3), 343–350. https://doi.org/10.1001/jamaoncol.2018.5080.
Seligmann, J. F., Fisher, D., Elliott, F., Richman, S., Butler, R., et al. (2015). Exploring the poor outcomes of BRAF mutant (BRAF mut) advanced colorectal cancer (aCRC): Analysis from 2,530 patients (pts) in randomized clinical trials (RCTs). Journal of Clinical Oncology, 33(15_suppl), 3509. https://doi.org/10.1200/jco.2015.33.15_suppl.3509.
Morris, V., Overman, M. J., Jiang, Z. Q., Garrett, C., Agarwal, S., Eng, C., Kee, B., Fogelman, D., Dasari, A., Wolff, R., Maru, D., & Kopetz, S. (2014). Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clinical Colorectal Cancer, 13(3), 164–171. https://doi.org/10.1016/j.clcc.2014.06.001.
Maughan, T. S., Adams, R. A., Smith, C. G., Meade, A. M., Seymour, M. T., Wilson, R. H., Idziaszczyk, S., Harris, R., Fisher, D., Kenny, S. L., Kay, E., Mitchell, J. K., Madi, A., Jasani, B., James, M. D., Bridgewater, J., Kennedy, M. J., Claes, B., Lambrechts, D., Kaplan, R., et al. (2011). Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet (London, England), 377(9783), 2103–2114. https://doi.org/10.1016/S0140-6736(11)60613-2.
Bokemeyer, C., Van Cutsem, E., Rougier, P., Ciardiello, F., Heeger, S., Schlichting, M., Celik, I., & Köhne, C. H. (2012). Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. European journal of cancer (Oxford, England : 1990), 48(10), 1466–1475. https://doi.org/10.1016/j.ejca.2012.02.057.
Stintzing, S., Miller-Phillips, L., Modest, D. P., Fischer von Weikersthal, L., Decker, T., Kiani, A., Vehling-Kaiser, U., Al-Batran, S. E., Heintges, T., Kahl, C., Seipelt, G., Kullmann, F., Stauch, M., Scheithauer, W., Held, S., Moehler, M., Jagenburg, A., Kirchner, T., Jung, A., Heinemann, V., et al. (2017). Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: Analysis of the FIRE-3 (AIO KRK-0306) study. European journal of cancer (Oxford, England : 1990), 79, 50–60. https://doi.org/10.1016/j.ejca.2017.03.023.
Geissler, M., Riera-Knorrenschild, J., Tannapfel, A., Greeve, J., Florschütz, A., Wessendorf, S., et al. (2018). mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): A randomized phase II VOLFI trial of the AIO (AIO-KRK0109). Journal of Clinical Oncology, 36(15_suppl), 3509. https://doi.org/10.1200/JCO.2018.36.15_suppl.3509.
Scott Kopetz, Axel Grothey, Rona Yaeger, Eric Van Cutsem, Jayesh Desai, Takayuki Yoshino, Harpreet Wasan, Fortunato Ciardiello, Fotios Loupakis, Yong Sang Hong, Neeltje Steeghs, Tormod K. Guren, Hendrik-Tobias Arkenau, Pilar Garcia-Alfonso, Per Pfeiffer, Sergey Orlov, Sara Lonardi, Elena Elez, Tae-Won Kim, Jan H.M. Schellens, Christina Guo, Asha Krishnan, Jeroen Dekervel, Van Morris, Aitana Calvo Ferrandiz, L.S. Tarpgaard, Michael Braun, Ashwin Gollerkeri, Christopher Keir, Kati Maharry, Michael Pickard, Janna Christy-Bittel, Lisa Anderson, Victor Sandor, Josep Tabernero, (2019). Encorafenib, Binimetinib, and Cetuximab in V600E–Mutated Colorectal Cancer. The New England Journal of Medicine 381(17):1632–1643.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Gábor Lakatos: honoraria as speaker received by: Roche, Merck, Amgen, Celgene, Bayer, Sanofi-Aventis (2015–2020)
Claus-Henning Köhne: honoraria as speaker by Merck, Amgen, Lilly, Bayer, Servier, Janssen. Advosiry board member of Merck, Servier, Bayer.
György Bodoky: advisory board member and honoraria as speaker of Roche, Servier, Bayer, Novartis, Pfizer, Celgene, Janssen, Amgen, Ipsen, Taiho, Merck.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakatos, G., Köhne, CH. & Bodoky, G. Current therapy of advanced colorectal cancer according to RAS/RAF mutational status. Cancer Metastasis Rev 39, 1143–1157 (2020). https://doi.org/10.1007/s10555-020-09913-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09913-7