Abstract
In the past years, a multitude of studies has been published in the field of pancreatic organogenesis to interrogate the critical regulators of endocrine lineage segregation. Preliminary, transcription factors are guiding the transcriptional hierarchy of the endocrine specified cells, underpinning the importance of open chromatin formation. Signaling pathways either inhibit or accelerate the transcriptional landscape of pancreatic organogenesis. Thus, the fine-tuned process in the former pancreatic multipotent progenitors in the mechanism of lineage segregation needs to be elucidated more precisely for unraveling the temporal-spatial lineage-determining factors.
Previously, Willmann et al. described candidate gene regulators of lineage segregation during the secondary transition of pancreatic organogenesis. At embryonic stage (E) 12.5, the former multipotent pancreatic progenitor compartmentalizes into the acinar, ductal, and endocrine lineage. In the adult pancreatic gland, acinar cells secrete enzymes that are transported by the duct to the duodenum. In contrast, the endocrine cells are clustered within the acinar tissue in the Islets of Langerhans. These Islets of Langerhans consist of a subset of α, δ, ε, and PP cells and β cells, and the function of the α and β cells is predominantly described by regulating glucose homeostasis, contrary, the function of the additional subtypes in the Islets of Langerhans remains still unclear and is rather pointing to a supportive role for the α and β cells.
The essential wave of endocrine precursor cells emerges at E 14.5 out of the ductal cord-like structure in a process called epithelial-to-mesenchymal transition (EMT). This EMT is a reversible and incomplete process that includes significant intermedia states. As EMT is in focus in the field of cancer research, missense in endocrine lineage segregation is linking to a progression of pancreatic cancer, to be more precise in adenocarcinoma, e.g., meaning pancreatic ductal adenocarcinoma.
Thus, the previous review will further accelerate the understanding of EMT about endocrine lineage segregation, respective pancreatic ductal adenocarcinoma, and introduces factors previously only known for either lineage segregation or related in cancer disease into a complete picture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
The article summarizes a review of the current literature of pancreatic lineage allocation into the endocrine lineage, represented by the gene Synaptotagmin 13. Furthermore, a descriptive link of endocrine lineage allocation and cancerogenesis is described with a focus on Synaptotagmin 13 and one more potential candidate 533041C22Rik, which is incorporated in the discussion.
2 Pancreatic lineage segregation—classical key regulators
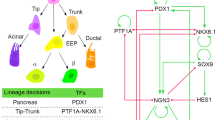
The key transcription factor in pancreatic organogenesis can be considered the Pdx1 gene, and lineage-tracing experiments demonstrate clearly that pancreatic progenitors are expressing the Pdx1 result in the acinar, ductal, and endocrine cells in the mature pancreatic gland [3]. To which extend the upstream regulator of Pdx1, Foxa2, is involved in pancreatic organogenesis remains still elusive. Still, preliminary results may hint to a cooperatively driven transcriptional hierarchy of Foxa2 and Pdx1 in the fine-tuned pancreatic initiation at approximately embryonic day (E) 8.5 (unless stated otherwise, the embryonic stages refer to mouse embryonic development). Here precise regulation on a protein level further determines the lineages of the pancreatic progenitors and thereby pointing to a non-reversible established lineage of the different pancreatic progenitor cells instead of the speculated multipotency character in the pancreatic epithelium [21]. Thus, a subpopulation of a Pdx1high directing the progenitors into ductal inherited cells consisting of ductal and endocrine progenitors, whereas the subpopulation of Pdx1low inherits the acinar progenitor pool. This acinar progenitor pool is mainly characterized by the genes Ptf1α, Cpa1, and Nr5a2 in which after the so-called first transition, at approximately E 9.5, these genes are expressed in the pancreatic epithelium and become later restricted in the secondary transition to the periphery, respective tip region of the evolving pancreas [12].
The main focus in pancreatic development is on endocrine lineage segregation as these cells will finally lead to the ß cell in the mature Islets of Langerhans. Thus, the ductal cord at E 14.5 persists of the bipotent progenitor’s preliminary marked by the gene Sox9 located in the central region of the pancreas, the so-called trunk pattern. Also, recently published, Sox9 is a critical player in ductal-derived endocrine cell differentiation in the pancreatic organogenesis, and the gene itself suggests not to contribute to endocrine cells in the mature gland. This trunk pattern inherits next to Sox9 an Ngn3-transient cell population, thereby representing the endocrine progenitors. Lineage tracing experiments implicated for Ngn3 as an essential helix-loop-helix transcription factor the necessity for the segregation of endocrine progenitors into the endocrine precursors as a downstream regulator of Sox9 [2, 15, 16].
Furthermore, Lynn and Seymour proposed a cell-autonomous role for Sox9 in Ngn3 induction, which suggests a negative feedback-loop for co-related expression of Sox9 and Ngn3 [11, 17, 18]. This result may further determine the importance of the gene Ngn3 in endocrine precursor segregation, especially regarding the upstream regulators Sox9 and Pdx1high. The signal cascade for the regulation of Ngn3 is still controversial, as there might be extrinsic and intrinsic signals that affect the proliferation of the endocrine lineage. Interestingly, signals from mesenchyme might not play a role in endocrine formation—as the proximity of the pancreatic epithelium to the mesenchyme accelerates the exocrine fate, whereas missing contact of the epithelium to the mesenchyme results in the endocrine lineage [10].
The entry of the endocrine precursors is marked by the family of NKx6 transcription factor genes (Nkx6.1 and Nkx6.2). Thereby, Pdx1high expression correlates with Nkx6 transcription factor expression, suggesting an activation of Nkx6.1 and Nkx6.2 by a complex mainly characterized by an abundance of a certain level of the protein Pdx1. Also, phenotype analysis of mouse mutants does reveal an absent of ß cells for Nkx6.1 and respective α cells for Nkx6.2, these genes are not essential for proper pancreatic organ formation [4, 5, 14, 20]. Thus, these results may point that the endocrine precursors are already primed for the final lineage in the subpopulation of the immature Islets of Langerhans.
3 Pancreatic ductal adenocarcinoma cancer factors—classical key regulators
The pancreatic ductal adenocarcinoma is a highly fatal disease because therapies against this tumor are ineffectively due to the metastatic stage, e.g., by a diagnosis of pancreatic ductal adenocarcinoma cancer factors (PDAC), the progression of the cancer is already spawned. Thus, the focus is on early diagnosis by the use of biomarkers for determining the state of occurrence of the disease. This neoplasia is described in the context of a progression model, wherein the ductal epithelium undergoes remodeling into PDAC through a series of so-called pancreatic intraepithelial neoplasia (PanIN), which are histologically defined precursors. Also, the PanINs are characterized by upregulated gene expression of Her-2 and K-ras (PanIN-1A and PanIN-1B), p16 (PanIN-2) and p53, and DPC4 and BRCA2 in PanIN-3; the review of Hruban and colleagues nicely depicts the essential pathway members and illustrates the model [6]. Contrary, the result only points to the descriptive role of overexpression of these genes in the context of neoplasia in the pancreatic ductal epithelium. Further data are lacking, especially given the mechanistically understanding.
4 Pancreatic cancerogenesis and endocrine lineage segregation by mechanistic regulation through EMT
The classical model of EMT, already described in the context of cancerogenesis, suggests to depict the gatekeepers and hallmarks of pancreatic cancer progression. Moreover, as the mechanism of EMT is proposed in endocrine lineage segregation, deciphering novel factors may shed light into the intermediate states and the factors either stabilizing these states or accelerating the state of progenitors, respective precursors.
As the endocrine precursors emerge out of the ductal cord, the association of PDAC and endocrine lineage segregation about the mechanism EMT is clear by sight. We illustrate here current models in the process of delamination of cells out of the ductal cord. In this process, epithelial ductal inherited cells, either marked by a Ngn3low or a Ngn3high subpopulation stepwise, change the cell polarity into the acquisition of motility properties. The exact intermediate states of the formerly endocrine progenitors into endocrine precursors are poorly understood but may involve Nkx6 transcription family members. Thus, we assume an Nkx6.1, respective Nkx6.2 expressing precursor cells already represents an intermediate lineage determined endocrine cell. Notably, ductal neoplasia is characterized by the clonal growth of a single cell and later resulting in waves of clonal expansion represented here in Fig. 1 under (1) single-cell delamination. Under the perspective of the genetic progression model, at PanIN-3 stage, cells delaminate and will spawn later into the surrounding tissue, vessels, and neighboring tissue. Under the perspective of the occurrence of Nkx6 intermediate lineage-determining stages, an underlying EMT mechanism, as proposed by Fig. 1 under (2) clustered cell delamination, suggests to more likely represent the endocrine lineage segregation rather than single-cell delamination under Fig. 1 (1). Therefore, we propose a model in which the endocrine precursors are already pre-committed and subsequently leave the ductal cord in a temporal-spatial manner and congregate to the architectural structure of the pre-defined immature Islets of Langerhans in mice.
The first model instead suggests single-cell delamination in the context of cancer progression, respective PDAC in pancreatic organogenesis ((1) Single-cell delamination). Contrary, the second model, already proposed by Pictet et al. in 1972, illustrates stepwise delamination of the pancreatic progenitors and/or precursors and aggregation in of these cells in the pancreatic epithelium at lineage segregation during organogenesis between the E 14.5 and E 16.5.
5 Regulators of pancreatic lineage segregation in cancerogenesis progression
Recently, Roy and colleagues illustrated that Pdx1 suggests regulating PDAC initiation and maintenance dynamically [13]. Preliminary, they investigated the tumor suppression and the oncogenic activity of Pdx1 in the pancreatic exocrine gland. Furthermore, a subset of cancer cells undergoing EMT reveals reduced to none expression of the pancreatic epithelial marker Pdx1. Nevertheless, Pdx1 may suggest being essential for the cancer progression in the pancreatic ductal lesion, either in acinar-to-ductal metaplasia (ADM) or PDAC. Thus, pointing to an intermediate state in the process of EMT and mechanistically different regulations of endocrine lineage segregation versus cancer progression. Taken the model from above (Fig. 1, (1) Single-cell delamination), single-cell delamination also illustrates the change in front-rear polarity by the depicted nucleus and thereby loss of the established epithelial polarity. Contrary, the model of endocrine precursors suggests at least a shift of polarity. However, the clustered cell delamination may hint to a still maintained elementary polarity for clustered cells to emerge out of the ductal cord into the immature Islets of Langerhans in an already defined architectural structure (Fig. 1, (2) Clustered cell delamination). In line with the previous illustrated results of Pdx1 in PDAC are results of an upstream regulator of Pdx1. The gene Foxa2 is a critical regulator of EMT, and thus loss-and-gain studies revealed suppressed expression in the process of EMT by break-down of adherents junctions via regulation of E-cadherin, but Foxa2 expression is re-activated in well-differentiated cancer cells.
Interestingly, the regulatory gene of acinar lineage segregation, Ptf1α, may play a role in oncogenic suppression of the PDAC, to be more precise in the PanIN of the ductal inherited cells and thus an expression of the gene Ptf1α is downregulated in invasive acinar PDACs [7]. Also, these results might suggest a partial EMT mechanism of the pancreatic epithelial cells in the process of lineage segregation and taken this hypothesis, the multipotency of the pancreatic epithelium is mainly guided through signaling pathways, e.g., Tgfβ or Wnt/PCP members as Frizzled (Frz) or Dishevelled (Dsh), activating or repressing junctional arrangement by use of the classically known lineage segregation factors. Therefore, the pancreatic epithelium in organogenesis does not per se have an established polarity, either in the acinar, ductal, or endocrine progenitor lineage, and with this in mind, the polarity determines the lineage rather than the transcriptional hierarchy.
Furthermore, the gene Nr5a2, after secondary transition specifically regional expressed in the tip region of the pancreatic epithelium, is described in the context of cancer progression as being upregulated and suggests to inhibiting EMT and thus, inhibit cancer progression in the pancreas [7] whereas Sox9 is directly linked to PDAC, but only present in a minor subpopulation of approximately 0.8% and not determined for the PDAC progenitor of PanINs [19]. Earlier published by Kopp et al. PanIN2/3 are represented by heterogenous Sox9 expression and thereby associated with acinar-derived PDAC initiation. In KrasG12D mice, Sox9 was detected in the acinar tissue pointing to a concomitant of PDAC initiation by Sox9 and KrasG12D missense expression through suppression of the acinar lineage determinants. Also, several ductal genes are further promoted, but the suppressed acinar tissue is not capable of being reprogrammed into ductal progenitors. As a result, an ADM does not necessarily depend on Sox9 expression, but Sox9 suggests to be essential in the stages of the progenitor stages of the ADM, the PanINs [8]. With this in mind, Ngn3, downstream of Sox9, is described only in the context of being repressed by Zinc Finger E-Box Binding Homeobox 1 (Zeb1), whereas Zeb1 initiates a tumorigenic phenotype, likely because a partial EMT is induced. Further experimental designs in regard to Ngn3 as a potential player in the initiation of PanINs will shed light on the partial EMT mechanism and the differences between endocrine versus cancerogenesis in this mechanism [22].
The family of Nkx6 genes is rarely described in the context of cancerogenesis. Cervical cancer cell lines are used for determining the absence of Nkx6.1 accompanied by the absence of the junctional polarity established by E-cadherin. Furthermore, mesenchymal properties, as stated by Vimentin expression, are downregulated in Nkx6.1 stable transfected Hela and/or SiHa cells with the conclusion by the authors of repressing cancer invasion by accelerating expression of epithelial markers [9]. We further suggest that Nkx6.1 instead not suppresses EMT but authorizes a partial EMT, which differs from the classical-known mechanism of EMT regarding cancerogenesis. Thus, we refer to the different models proposed for the mechanism of EMT and suggest only a partial loss of polarity in the process of endocrine formation. Further experiments would be sufficient for prefiguring the involvement of the mechanism of EMT in endocrine progenitors as Ngn3 and/or subsequent endocrine precursors as Nkx6.1, respective Nkx6.2.
6 Conclusion
6.1 Further perspectives of novel putative lineage segregation factor and the relation to PDAC/PanIN
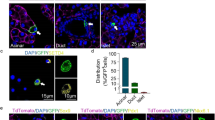
Recently published, Synaptotagmin 13 suggests being involved in the process of endocrine lineage segregation, as preliminary results point to concomitant expression of Foxa2, in particular, Foxa2high, respective Pdx1high, and Syt13 [21]. The precise function of Syt13 remains unclear, and Syt13 may instead be involved in vesicle-mediated transport, e.g., as a scaffold protein in trafficking within a cell, because typical exocytotic properties of the Synaptotagmin gene in the amino acid structure are lacking in Syt13. As observed in the pancreatic paraffin section of 3-month mutant mice, Syt13 hint to the process of cell delamination in the mechanism of EMT. By using a gene trap allele, including an incorporated lacZ-reporter cassette in the floxed allele of the mutant mice, Syt13 expression can be followed by staining for β-galactosidase (kindly provided by EUCOMM). Thus, the reporter construct illustrates in Fig. 2, the representative staining in blue color in the Islets of Langerhans, identified by the confined cells by dense aggregation. Furthermore, the pancreas illustrates pancreatic lesions, categorized into PanIN 2, as the cells lining the lesion represent a monolayer. Taken these preliminary results, the Syt13 mutant mouse model suggests being a suitable model for mimicking the mechanism of EMT in endocrine lineage segregation and the process of cancerogenesis, in particular, the PanINs and in later stages, the PDAC-derived tumors. The partial EMT in both processes may be investigated more precisely for evaluating the different signaling factors for either choice and, in particular, for the partial EMT.
On the left side, PanIN and PDAC illustrated as ductile-derived cancerogenesis lesions of the pancreas. A monolayer characterizes the PanIN, wherein the cells of the monolayer lining the PanIN have a nucleus in the still orientated stable polarity complex. Contrary, the PDAC illustrates a multilayer of cells, wherein the cells are representing nuclei differentially distributed within the cells, and thus, invasive characteristics. In the pancreatic section of 3-month mice of the Syt13 mutant, the PanIN is indicated by the black lines.
On the right side, the ductal-derived endocrine precursors are orientated in a pre-defined manner in the Islets of Langerhans and on the pancreatic section of the 3-month mice of the Syt13 mutant indicated by the black lines. Furthermore, the duct is indicated by the black lines on the paraffin section. The pancreatic section illustrates that the pancreatic lesion and the Islets of Langerhans are ductal-derived by a mechanism proposed as EMT. Furthermore, a factor involved in cancerogenesis and endocrine progenitor, respective precursor lineage segregation, suggests being Syt13.
In addition, the gene 533041C22 Rik may be a promising target for further elucidating factors essential for partial EMT. The gene itself is already described in the context of the gene maba-1 (also known as KIAA1324), and the expression level of maba-1 suggests of guiding metastasis and tumor progression in breast and lung cancer cell lines [1]. Also, knockdown of maba-1 on an mRNA level and an antibody inhibits progression of cancer, e.g., the proliferation of HCT116 colon cancer cells (EPO Global Dossier US 64783309), and thus, pointing to a role of maba-1 in remodeling an epithelial cell into a cell capable of being invasive and migratory. Also, preliminary results may hint to maba-1 as a factor in endocrine lineage segregation, which as described above, could be a suitable factor for determining the partial EMT, especially in regard of the convention and the differences of the mechanism of EMT in view of endocrine lineage segregation and cancer progression.
Thus, two putative novel genes in endocrine lineage segregation reveal a direct and inevitable link to cancerogenesis. Furthermore, as endocrine progenitors are inherited in the ductal cord during pancreatic organogenesis, these two putative endocrine lineage segregation factors may play a role in the mechanism of EMT and thereby suggest to be investigated closer in regard to the key partial EMT which differentiates the mesenchymal-derived cancerogenesis cell from the endocrine precursor cell.
Data availability
The datasets used and/or analyzed during the current review are available from the corresponding author and are made released to the public under URN: urn:nbn:de:bvb:91-diss-20160711-1278135-1-9.
Change history
10 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10555-021-09978-y
Abbreviations
- ADM:
-
acinar-to-ductal metaplasia
- BRCA2:
-
BRCA2 DNA Repair Associated
- Cpa1:
-
Carboxypeptidase A1
- DCP4:
-
Decapping Enzyme 4
- Dsh:
-
Dishevelled
- E:
-
embryonic stage
- EMT:
-
epithelial-to-mesenchymal transition
- Foxa2:
-
Forkhead box 2
- Frz:
-
Frizzled
- Her-2:
-
Erb-B2 Receptor Tyrosine Kinase 2
- K-ras:
-
KRAS Proto-Oncogene
- Maba-1:
-
KIAA1324
- Ngn3:
-
Neurogenin 3
- Nkx6:
-
NK6 Homeobox 2
- Nr5a2:
-
Nuclear Receptor Subfamily 5 Group A Member 2
- P16:
-
protein 16
- P53:
-
protein 53
- PanIN:
-
pancreatic intraepithelial neoplasia
- PCP:
-
planar cell polarity
- PDAC:
-
pancreatic ductal adenocarcinoma
- Pdx1:
-
pancreatic and duodenal homeobox 1
- PP:
-
pancreatic polypeptide cells
- Ptf1α:
-
Pancreas Associated Transcription Factor 1a
- Sox9:
-
SRY-Box Transcription Factor 9
- Syt13:
-
Synaptotagmin 13
- Tgfβ:
-
transforming growth factor beta
- Wnt:
-
wingless
- Zeb1:
-
Zinc Finger E-Box Binding Homeobox 1
- α:
-
alpha cells
- β:
-
insulin-producing cells
- δ:
-
delta cells
- ε:
-
etha cells
References
Bauer, M., Aust, H., & Schuhmacher, U. (2004). Different transcriptional expression of KIAA1324 and its splicing variants in human carcinoma cell lines with different metastatic capacity. Oncol Rep., 11(3), 677–680.
Dubois, C. L., Shih, H. P., Seymour, P. A., Patel, N. A., Behrmann, J. M., Ngo, V., & Sander, M. (2011). Sox9-haploinsufficiency causes glucose intolerance in mice. PLoS ONE, 6(8), e23131.
Gu, G. (2004). Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development, 131(1), 165–179. https://doi.org/10.1242/dev.00921.
Habener, J. F., Kemp, D. M., & Thomas, M. K. (2005). Minireview: transcriptional regulation in pancreatic development. Endocrinology., 146, 1025–1034.
Henseleit, K. D., Nelson, S. B., Kuhlbrodt, K., Hennings, J. C., Ericson, J., & Sander, M. (2005). NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development (Cambridge, England), 132(13), 3139–3149. https://doi.org/10.1242/dev.01875.
Hruban, R. H., Goggins, M., Parsons, J., & Kern, S. E. (2000). Progression model for pancreatic cancer progression model for pancreatic cancer 1, 6(August), 2969–2972.
Krah, N. M., De La, O. J. P., Swift, G. H., Hoang, C. Q., Willet, S. G., Pan, F. C., et al. (2015). The acinar differentiation determinant PTF1A inhibits initiation of pancreatic ductal adenocarcinoma. eLife, 4(JULY2015), 1–25. https://doi.org/10.7554/eLife.07125.001.
Kopp, J. L., von Figura, G., Mayes, E., Liu, F-F., Dubois, C. L., Morris, J. P., Pan, F. C., Akiyama, H., Wright, C. V. E., Jensen, K., Hebrok, M., Sander M. (2012). Identification of Sox9-dependent Acinar-to-ductal Reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cell press, 22(6), 737–750. https://doi.org/10.1016/j.ccr.2012.10.025.
Li, H. J., Yu, P. N., Huang, K. Y., Su, H. Y., Hsiao, T. H., Chang, C. P., Yu, M. H., & Lin, Y. W. (2016). NKX6.1 functions as a metastatic suppressor through epigenetic regulation of the epithelial-mesenchymal transition. Oncogene, 35(17), 2266–2278. https://doi.org/10.1038/onc.2015.289.
Li, Z., Manna, P., Kobayashi, H., Spilde, T., Bhatia, A., Preuett, B., Prasadan, K., Hembree, M., & Gittes, G. K. (2004). Multifaceted pancreatic mesenchymal control of epithelial lineage selection. Developmental Biology, 269(1), 252–263. https://doi.org/10.1016/j.ydbio.2004.01.043.
Lynn, F. C., Smith, S. B., Wilson, M. E., Yang, K. Y., Nekrep, N., & German, M. S. (2007). Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 104(25), 10500–10505. https://doi.org/10.1073/pnas.0704054104.
Pan, F. C., & Wright, C. (2011). Pancreas organogenesis: from bud to plexus to gland. Developmental Dynamics., 240, 530–565.
Roy, N., Takeuchi, K. K., Ruggeri, J. M., Bailey, P., Chang, D., Li, J., Leonhardt, L., Puri, S., Hoffman, M. T., Gao, S., Halbrook, C. J., Song, Y., Ljungman, M., Malik, S., Wright, C. V. E., Dawson, D. W., Biankin, A. V., Hebrok, M., & Crawford, H. C. (2016). PDX1 dynamically regulates pancreatic ductal adenocarcinoma initiation and maintenance. Genes and Development, 30(24), 2669–2683. https://doi.org/10.1101/gad.291021.116.
Schaffer, A. E., Taylor, B. L., Benthuysen, J. R., Liu, J., Thorel, F., Yuan, W., et al. (2013). Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic beta cell identity. PLoS Genetics, 9(1). https://doi.org/10.1371/journal.pgen.1003274.
Seymour, P. A. (2014). Sox9: a master regulator of the pancreatic program. Review of Diabetic Studies., 11, 51–83. https://doi.org/10.1900/RDS.2014.11.51.
Seymour, P. A., Freude, K. K., Dubois, C. L., Shih, H., Patel, N. A., & Sander, M. (2008). A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Developmental Biology, 323(1), 19–30. https://doi.org/10.1016/j.ydbio.2008.07.034.
Seymour, P. A., Shih, H. P., Patel, N. A., Freude, K. K., Xie, R., Lim, C. J., & Sander, M. (2012). A Sox9 / Fgf feed-forward loop maintains pancreatic organ identity., 3372, 3363–3372. https://doi.org/10.1242/dev.078733.
Shih, H. P., Wang, A., & Sander, M. (2013). Pancreas organogenesis: from lineage determination to morphogenesis. Annual Review of Cell and Developmental Biology, (July), 1–25. https://doi.org/10.1146/annurev-cellbio-101512-122405.
Tanaka, T., Omote, R., Okazaki, N., Yanai, H., Yoshino, T. (2017). Gastric neuroendocrine tumor arising from heterotopic pancreas. Clin J Gastroenterol, 11(1), 34–37. https://doi.org/10.1007/s12328-017-0795-3.
Taylor, B., Liu, F. F., & Sander, M. (2013). Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Reports, 4(6). https://doi.org/10.1016/j.celrep.2013.08.010.
Willmann, S. J., Mueller, N. S., Engert, S., Sterr, M., Burtscher, I., Raducanu, A., Irmler, M., Beckers, J., Sass, S., Theis, F. J., & Lickert, H. (2016). The global gene expression profile of the secondary transition during pancreatic development. Mechanisms of Development, 139, 51–64.
Zhou, C., Jiang, H., Zhang, Z., Zhang, G., Wang, H., Zhang, Q., Sun, P., Xiang, R., & Yang, S. (2017). ZEB1 confers stem cell-like properties in breast cancer by targeting neurogenin-3. Oncotarget, 8(33), 54388–54401. https://doi.org/10.18632/oncotarget.17077.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The author declares her own contribution.
Corresponding author
Ethics declarations
Competing interests
The author declares that there are no competing interests.
Ethical approval
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Willmann, S.J. Cutting the edge between cancerogenesis and organogenesis of the pancreatic endocrine lineage allocation—comprehensive review of the genes Synaptotagmin 13 and 533041C22 Rik in epithelial-to-mesenchymal transition. Cancer Metastasis Rev 39, 953–958 (2020). https://doi.org/10.1007/s10555-020-09897-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09897-4