Abstract
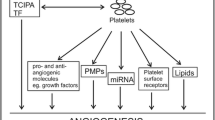
Platelets are cytoplasmic fragments generated by megakaryocytes in the bone marrow and do not possess a nucleus. They contribute to the “Circulome” consisting of all circulating cells, factors and macromolecules such as cfDNA. Their primary function is to recognize vascular lesions and initiate thrombus formation that ceases bleeding. This distinctive characteristic of platelets also contributes to cancer and its progression. The ability of platelets to recognize and interact with other cells and neighboring platelets enables them to interact with tumor cells in the circulation. Receptor recognition and factor mediated crosstalk between tumor cells and platelets stimulate platelet activation, release of factors, and aggregation that promotes tumor cell survival and cancer progression. This review describes platelet: (i) contributions to the “Circulome” (ii) their importance as diagnostic tools in predicting cancer risk and (iii) therapies targeting platelet activation in inhibiting tumor progression and metastasis.

Similar content being viewed by others
References
Zernecke, A., Bidzhekov, K., Noels, H., Shagdarsuren, E., Gan, L., Denecke, B., et al. (2009) Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Science Signaling, 2(100) ra81 doi:10.1126/scisignal.2000610.
Ribeiro, M. F., Zhu, H., Millard, R. W., & Fan, G. C. (2013). Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes, 2(1), 54–59. doi:10.2174/22115528113020020001.
Zhang, Y., Liu, D., Chen, X., Li, J., Li, L., Bian, Z., et al. (2010). Secreted monocytic miR-150 enhances targeted endothelial cell migration. Molecular Cell, 39(1), 133–144. doi:10.1016/j.molcel.2010.06.010.
Pulliam, L., & Gupta, A. (2015). Modulation of cellular function through immune-activated exosomes. DNA and Cell Biology, 34(7), 459–463. doi:10.1089/dna.2015.2884.
Rak, J. (2013). Extracellular vesicles - biomarkers and effectors of the cellular interactome in cancer. Frontiers in Pharmacology, 4, 21. doi:10.3389/fphar.2013.00021.
Azmi, A. S., Bao, B., & Sarkar, F. H. (2013). Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Reviews, 32(3–4), 623–642. doi:10.1007/s10555-013-9441-9.
Zhang, J., Li, S., Li, L., Li, M., Guo, C., Yao, J., et al. (2015). Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics, Proteomics & Bioinformatics, 13(1), 17–24. doi:10.1016/j.gpb.2015.02.001.
Lopes-Rodrigues, V., Di Luca, A., Sousa, D., Seca, H., Meleady, P., Henry, M., et al. (2016). Multidrug resistant tumour cells shed more microvesicle-like EVs and less exosomes than their drug-sensitive counterpart cells. Biochimica et Biophysica Acta, 1860(3), 618–627. doi:10.1016/j.bbagen.2015.12.011.
Ciardiello, C., Cavallini, L., Spinelli, C., Yang, J., Reis-Sobreiro, M., de Candia, P., et al. (2016). Focus on extracellular vesicles: new frontiers of cell-to-cell communication in cancer. International Journal of Molecular Sciences, 17(2). doi:10.3390/ijms17020175.
Yu, S., Cao, H., Shen, B., Feng, J. (2015). Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget, 6(35), 37151–37168, doi:10.18632/oncotarget.6022.
Davila, M., Robles-Carrillo, L., Unruh, D., Huo, Q., Gardiner, C., Sargent, I. L., et al. (2014). Microparticle association and heterogeneity of tumor-derived tissue factor in plasma: is it important for coagulation activation? Journal of Thrombosis and Haemostasis, 12(2), 186–196. doi:10.1111/jth.12475.
Melo, S. A., Luecke, L. B., Kahlert, C., Fernandez, A. F., Gammon, S. T., Kaye, J., et al. (2015). Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. doi:10.1038/nature14581.
Thakur, B. K., Zhang, H., Becker, A., Matei, I., Huang, Y., Costa-Silva, B., et al. (2014). Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Research, 24(6), 766–769. doi:10.1038/cr.2014.44.
Botti, G., Marra, L., Malzone, M. G., Anniciello, A., Botti, C., Franco, R., et al. (2017). LncRNA HOTAIR as prognostic circulating marker and potential therapeutic target in patients with tumor diseases. Current Drug Targets. 18(1), 27-34.
Fesler, A., Jiang, J., Zhai, H., & Ju, J. (2014). Circulating microRNA testing for the early diagnosis and follow-up of colorectal cancer patients. Molecular Diagnosis & Therapy, 18(3), 303–308. doi:10.1007/s40291-014-0089-0.
Huang, X., Yuan, T., Tschannen, M., Sun, Z., Jacob, H., Du, M., et al. (2013). Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics, 14, 319. doi:10.1186/1471-2164-14-319.
Khan, N., Mironov, G., & Berezovski, M. V. (2016). Direct detection of endogenous microRNAs and their post-transcriptional modifications in cancer serum by capillary electrophoresis-mass spectrometry. Analytical and Bioanalytical Chemistry. doi:10.1007/s00216-015-9277-y.
Silva, A., Bullock, M., & Calin, G. (2015). The clinical relevance of long non-coding RNAs in cancer. Cancers (Basel), 7(4), 2169–2182. doi:10.3390/cancers7040884.
Mandel, P., & Metais, P. (1948). Not Available. Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales, 142(3–4), 241–243.
Heitzer, E., Ulz, P., & Geigl, J. B. (2015). Circulating tumor DNA as a liquid biopsy for cancer. Clinical Chemistry, 61(1), 112–123. doi:10.1373/clinchem.2014.222679.
Ulz, P., Auer, M., & Heitzer, E. (2016). Detection of circulating tumor DNA in the blood of cancer patients: an important tool in cancer chemoprevention. Methods in Molecular Biology, 1379, 45–68. doi:10.1007/978-1-4939-3191-0_5.
Warton, K., & Samimi, G. (2015). Methylation of cell-free circulating DNA in the diagnosis of cancer. Frontiers in Molecular Biosciences, 2, 13. doi:10.3389/fmolb.2015.00013.
Beljanski, M., & Plawecki, M. (1979). Particular RNA fragments as promoters of leukocyte and platelet formation in rabbits. Experimental Cell Biology, 47(3), 218–225.
Korosteleva, T. A., & Chistiakova, Z. M. (1979). Immunological study of rat liver RNA in the early stages of hepatic carcinogenesis. Voprosy Onkologii, 25(8), 77–81.
Lawrie, C. H., Gal, S., Dunlop, H. M., Pushkaran, B., Liggins, A. P., Pulford, K., et al. (2008). Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. British Journal of Haematology, 141(5), 672–675. doi:10.1111/j.1365-2141.2008.07077.x.
Chim, S. S., Shing, T. K., Hung, E. C., Leung, T. Y., Lau, T. K., Chiu, R. W., et al. (2008). Detection and characterization of placental microRNAs in maternal plasma. Clinical Chemistry, 54(3), 482–490. doi:10.1373/clinchem.2007.097972.
Spindler, K. L., Pallisgaard, N., Andersen, R. F., Brandslund, I., & Jakobsen, A. (2015). Circulating free DNA as biomarker and source for mutation detection in metastatic colorectal cancer. PloS One, 10(4), e0108247. doi:10.1371/journal.pone.0108247.
Bryzgunova, O. E., & Laktionov, P. P. (2015). Generation of blood circulating DNA: the sources, peculiarities of circulation and structure. Biomeditsinskaya Khimiya, 61(4), 409–426. doi:10.18097/PBMC20156104409.
Vasilyeva, L. N., Podgornaya, O. I., & Bespalov, V. G. (2015). Nucleosome fracton of extracellular dna as the index of apoptosis. Tsitologiia, 57(2), 87–94.
Wissler, J. H., Wissler, J. E., & Logemann, E. (2008). Extracellular functional noncoding nucleic acid bioaptamers and angiotropin RNP ribokines in vascularization and self-tolerance. Annals of the New York Academy of Sciences, 1137, 316–342. doi:10.1196/annals.1448.047.
Molnar, B., Toth, K., Bartak, B. K., & Tulassay, Z. (2015). Plasma methylated septin 9: a colorectal cancer screening marker. Expert Review of Molecular Diagnostics, 15(2), 171–184. doi:10.1586/14737159.2015.975212.
Patai, A. V., Valcz, G., Hollosi, P., Kalmar, A., Peterfia, B., Patai, A., et al. (2015). Comprehensive DNA methylation analysis reveals a common ten-gene methylation signature in colorectal adenomas and carcinomas. PloS One, 10(8), e0133836. doi:10.1371/journal.pone.0133836.
Hao, T. B., Shi, W., Shen, X. J., Qi, J., Wu, X. H., Wu, Y., et al. (2014). Circulating cell-free DNA in serum as a biomarker for diagnosis and prognostic prediction of colorectal cancer. British Journal of Cancer, 111(8), 1482–1489. doi:10.1038/bjc.2014.470.
Tie, J., Kinde, I., Wang, Y., Wong, H. L., Roebert, J., Christie, M., et al. (2015). Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Annals of Oncology, 26(8), 1715–1722. doi:10.1093/annonc/mdv177.
Menter, D. G., Tucker, S. C., Kopetz, S., Sood, A. K., Crissman, J. D., & Honn, K. V. (2014). Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Reviews, 33(1), 231–269. doi:10.1007/s10555-014-9498-0.
Lanman, R. B., Mortimer, S. A., Zill, O. A., Sebisanovic, D., Lopez, R., Blau, S., et al. (2015). Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PloS One, 10(10), e0140712. doi:10.1371/journal.pone.0140712.
Jovelet, C., Ileana, E., Le Deley, M. C., Motte, N., Rosellini, S., Romero, A., et al. (2016). Circulating cell-free tumor DNA (cfDNA) analysis of 50-genes by next-generation sequencing (NGS) in the prospective MOSCATO trial. Clinical Cancer Research. doi:10.1158/1078-0432.CCR-15-2470.
Kalia, M. (2015). Biomarkers for personalized oncology: recent advances and future challenges. Metabolism, 64(3 Suppl 1), S16–S21. doi:10.1016/j.metabol.2014.10.027.
Patel, K. M., & Tsui, D. W. (2015). The translational potential of circulating tumour DNA in oncology. Clinical Biochemistry, 48(15), 957–961. doi:10.1016/j.clinbiochem.2015.04.005.
Kensler, T. W., Spira, A., Garber, J. E., Szabo, E., Lee, J. J., Dong, Z., et al. (2016). Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prevention Research (Philadelphia, Pa.), 9(1), 2–10. doi:10.1158/1940-6207.CAPR-15-0406.
Russo, M., Siravegna, G., Blaszkowsky, L. S., Corti, G., Crisafulli, G., Ahronian, L. G., et al. (2016). Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discovery, 6(2), 147–153. doi:10.1158/2159-8290.CD-15-1283.
Santiago-Walker, A., Gagnon, R., Mazumdar, J., Casey, M., Long, G. V., Schadendorf, D., et al. (2016). Correlation of BRAF mutation status in circulating-free DNA and tumor and association with clinical outcome across four BRAFi and MEKi clinical trials. Clinical Cancer Research, 22(3), 567–574. doi:10.1158/1078-0432.CCR-15-0321.
Zill, O. A., Greene, C., Sebisanovic, D., Siew, L. M., Leng, J., Vu, M., et al. (2015). Cell-free DNA next-generation sequencing in pancreatobiliary carcinomas. Cancer Discovery, 5(10), 1040–1048. doi:10.1158/2159-8290.CD-15-0274.
Bratman, S. V., Newman, A. M., Alizadeh, A. A., & Diehn, M. (2015). Potential clinical utility of ultrasensitive circulating tumor DNA detection with CAPP-Seq. Expert Review of Molecular Diagnostics, 15(6), 715–719. doi:10.1586/14737159.2015.1019476.
Reinert, T., Scholer, L. V., Thomsen, R., Tobiasen, H., Vang, S., Nordentoft, I., et al. (2015). Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. doi:10.1136/gutjnl-2014-308859.
Thress, K. S., Brant, R., Carr, T. H., Dearden, S., Jenkins, S., Brown, H., et al. (2015). EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer, 90(3), 509–515. doi:10.1016/j.lungcan.2015.10.004.
Richardson, A. L., & Iglehart, J. D. (2012). BEAMing up personalized medicine: mutation detection in blood. Clinical Cancer Research, 18(12), 3209–3211. doi:10.1158/1078-0432.CCR-12-0871.
Castellanos-Rizaldos, E., Paweletz, C., Song, C., Oxnard, G. R., Mamon, H., Janne, P. A., et al. (2015). Enhanced ratio of signals enables digital mutation scanning for rare allele detection. The Journal of Molecular Diagnostics, 17(3), 284–292. doi:10.1016/j.jmoldx.2014.12.003.
Tost, J. (2016). The clinical potential of Enhanced-ice-COLD-PCR. Expert Review of Molecular Diagnostics, 16(3), 265–268. doi:10.1586/14737159.2016.1123623.
Yuan, H., Zhu, Z. Z., Lu, Y., Liu, F., Zhang, W., Huang, G., et al. (2012). A modified extraction method of circulating free DNA for epidermal growth factor receptor mutation analysis. Yonsei Medical Journal, 53(1), 132–137. doi:10.3349/ymj.2012.53.1.132.
Bell, D. A., & Morrison, B. (1991). The spontaneous apoptotic cell death of normal human lymphocytes in vitro: the release of, and immunoproliferative response to, nucleosomes in vitro. Clinical Immunology and Immunopathology, 60(1), 13–26.
Vasil'eva, I. N., & Zinkin, V. N. (2013). The value of low-molecular-weight DNA of blood plasma in the diagnostic of the patological processes of different genesis. Biomeditsinskaya Khimiya, 59(3), 358–373.
Wyllie, A. H. (1980). Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature, 284(5756), 555–556.
Chandrananda, D., Thorne, N. P., & Bahlo, M. (2015). High-resolution characterization of sequence signatures due to non-random cleavage of cell-free DNA. BMC Medical Genomics, 8, 29. doi:10.1186/s12920-015-0107-z.
Cai, X., Janku, F., Zhan, Q., & Fan, J. B. (2015). Accessing genetic information with liquid biopsies. Trends in Genetics, 31(10), 564–575. doi:10.1016/j.tig.2015.06.001.
Sequist, L. V., Soria, J. C., Goldman, J. W., Wakelee, H. A., Gadgeel, S. M., Varga, A., et al. (2015). Rociletinib in EGFR-mutated non-small-cell lung cancer. The New England Journal of Medicine, 372(18), 1700–1709. doi:10.1056/NEJMoa1413654.
Schiavon, G., Hrebien, S., Garcia-Murillas, I., Cutts, R. J., Pearson, A., Tarazona, N., et al. (2015). Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Science Translational Medicine, 7(313), 313ra182. doi:10.1126/scitranslmed.aac7551.
Schwarzenbach, H., Nishida, N., Calin, G. A., & Pantel, K. (2014). Clinical relevance of circulating cell-free microRNAs in cancer. Nature Reviews. Clinical Oncology, 11(3), 145–156. doi:10.1038/nrclinonc.2014.5.
Sun, Y., Liu, Y., Cogdell, D., Calin, G. A., Sun, B., Kopetz, S., et al. (2016). Examining plasma microRNA markers for colorectal cancer at different stages. Oncotarget. doi:10.18632/oncotarget.7196.
Igaz, I., & Igaz, P. (2015). Diagnostic relevance of microRNAs in other body fluids including urine, feces, and saliva. EXS, 106, 245–252. doi:10.1007/978-3-0348-0955-9_11.
Qin, J., Williams, T. L., & Fernando, M. R. (2013). A novel blood collection device stabilizes cell-free RNA in blood during sample shipping and storage. BMC Research Notes, 6, 380. doi:10.1186/1756-0500-6-380.
Zuo, Z., Maiti, S., Hu, S., Loghavi, S., Calin, G. A., Garcia-Manero, G., et al. (2015). Plasma circulating-microRNA profiles are useful for assessing prognosis in patients with cytogenetically normal myelodysplastic syndromes. Modern Pathology, 28(3), 373–382. doi:10.1038/modpathol.2014.108.
Alder, H., Taccioli, C., Chen, H., Jiang, Y., Smalley, K. J., Fadda, P., et al. (2012). Dysregulation of miR-31 and miR-21 induced by zinc deficiency promotes esophageal cancer. Carcinogenesis, 33(9), 1736–1744. doi:10.1093/carcin/bgs204.
Vickovic, S., Ahmadian, A., Lewensohn, R., & Lundeberg, J. (2015). Toward rare blood cell preservation for RNA sequencing. The Journal of Molecular Diagnostics, 17(4), 352–359. doi:10.1016/j.jmoldx.2015.03.009.
Liu, H. S., & Xiao, H. S. (2014). MicroRNAs as potential biomarkers for gastric cancer. World Journal of Gastroenterology, 20(34), 12007–12017. doi:10.3748/wjg.v20.i34.12007.
Mego, M., Cholujova, D., Minarik, G., Sedlackova, T., Gronesova, P., Karaba, M., et al. (2016). CXCR4-SDF-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. BMC Cancer, 16(1), 127. doi:10.1186/s12885-016-2143-2.
Wozniak, M. B., Scelo, G., Muller, D. C., Mukeria, A., Zaridze, D., & Brennan, P. (2015). Circulating MicroRNAs as non-invasive biomarkers for early detection of non-small-cell lung cancer. PloS One, 10(5), e0125026. doi:10.1371/journal.pone.0125026.
Grun, D., Lyubimova, A., Kester, L., Wiebrands, K., Basak, O., Sasaki, N., et al. (2015). Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature, 525(7568), 251–255. doi:10.1038/nature14966.
Zhang, J., Zhang, K., Bi, M., Jiao, X., Zhang, D., & Dong, Q. (2014). Circulating microRNA expressions in colorectal cancer as predictors of response to chemotherapy. Anti-Cancer Drugs, 25(3), 346–352. doi:10.1097/CAD.0000000000000049.
Pucciarelli, S., Rampazzo, E., Briarava, M., Maretto, I., Agostini, M., Digito, M., et al. (2012). Telomere-specific reverse transcriptase (hTERT) and cell-free RNA in plasma as predictors of pathologic tumor response in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Annals of Surgical Oncology, 19(9), 3089–3096. doi:10.1245/s10434-012-2272-z.
Verma, A. M., Patel, M., Aslam, M. I., Jameson, J., Pringle, J. H., Wurm, P., et al. (2015). Circulating plasma microRNAs as a screening method for detection of colorectal adenomas. Lancet, 385(Suppl 1), S100. doi:10.1016/S0140-6736(15)60415-9.
Kishikawa, T., Otsuka, M., Ohno, M., Yoshikawa, T., Takata, A., & Koike, K. (2015). Circulating RNAs as new biomarkers for detecting pancreatic cancer. World Journal of Gastroenterology, 21(28), 8527–8540. doi:10.3748/wjg.v21.i28.8527.
Lindner, K., Haier, J., Wang, Z., Watson, D. I., Hussey, D. J., & Hummel, R. (2015). Circulating microRNAs: emerging biomarkers for diagnosis and prognosis in patients with gastrointestinal cancers. Clinical Science (London, England), 128(1), 1–15. doi:10.1042/CS20140089.
Alsidawi, S., Malek, E., & Driscoll, J. J. (2014). MicroRNAs in brain metastases: potential role as diagnostics and therapeutics. International Journal of Molecular Sciences, 15(6), 10508–10526. doi:10.3390/ijms150610508.
Wulfken, L. M., Moritz, R., Ohlmann, C., Holdenrieder, S., Jung, V., Becker, F., et al. (2011). MicroRNAs in renal cell carcinoma: diagnostic implications of serum miR-1233 levels. PloS One, 6(9), e25787. doi:10.1371/journal.pone.0025787.
Pigati, L., Yaddanapudi, S. C., Iyengar, R., Kim, D. J., Hearn, S. A., Danforth, D., et al. (2010). Selective release of microRNA species from normal and malignant mammary epithelial cells. PloS One, 5(10), e13515. doi:10.1371/journal.pone.0013515.
Chakraborty, C., & Das, S. (2016). Profiling cell-free and circulating miRNA: a clinical diagnostic tool for different cancers. Tumour Biology. doi:10.1007/s13277-016-4907-3.
Lopez-Vilchez, I., Diaz-Ricart, M., Galan, A. M., Roque, M., Caballo, C., Molina, P., et al. (2016). Internalization of tissue factor-rich microvesicles by platelets occurs independently of GPIIb-IIIa, and involves CD36 receptor, serotonin transporter and cytoskeletal assembly. Journal of Cellular Biochemistry, 117(2), 448–457. doi:10.1002/jcb.25293.
Stone, R. L., Nick, A. M., McNeish, I. A., Balkwill, F., Han, H. D., Bottsford-Miller, J., et al. (2012). Paraneoplastic thrombocytosis in ovarian cancer. The New England Journal of Medicine, 366(7), 610–618. doi:10.1056/NEJMoa1110352.
Thon, J. N., & Italiano, J. E. (2010). Platelet formation. Seminars in Hematology, 47(3), 220–226. doi:10.1053/j.seminhematol.2010.03.005.
Agam, G., Bessler, H., & Djaldetti, M. (1976). In vitro DNA and RNA synthesis by human platelets. Biochimica et Biophysica Acta, 425(1), 41–48.
Macaulay, I. C., Carr, P., Gusnanto, A., Ouwehand, W. H., Fitzgerald, D., & Watkins, N. A. (2005). Platelet genomics and proteomics in human health and disease. The Journal of Clinical Investigation, 115(12), 3370–3377. doi:10.1172/jci26885.
Italiano Jr., J. E., & Shivdasani, R. A. (2003). Megakaryocytes and beyond: the birth of platelets. Journal of Thrombosis and Haemostasis, 1(6), 1174–1182.
Gnatenko, D. V., Dunn, J. J., Schwedes, J., & Bahou, W. F. (2009). Transcript profiling of human platelets using microarray and serial analysis of gene expression (SAGE). Methods in Molecular Biology, 496, 245–272. doi:10.1007/978-1-59745-553-4_16.
Denis, M. M., Tolley, N. D., Bunting, M., Schwertz, H., Jiang, H., Lindemann, S., et al. (2005). Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell, 122(3), 379–391. doi:10.1016/j.cell.2005.06.015.
Kieffer, N., Guichard, J., Farcet, J. P., Vainchenker, W., & Breton-Gorius, J. (1987). Biosynthesis of major platelet proteins in human blood platelets. European Journal of Biochemistry, 164(1), 189–195.
Arimori, S., & Sumitomo, K. (1978). Ultrastructural observation of openings of open canalicular system on the membrane surface of human platelet by freeze-etching method (author’s transl). Nihon Ketsueki Gakkai Zasshi, 41(3), 569–572.
White, J. G. (1972). Uptake of latex particles by blood platelets: phagocytosis or sequestration? The American Journal of Pathology, 69(3), 439–458.
Thon, J. N., & Italiano, J. E. (2012). Platelets: production, morphology and ultrastructure. Handbook of Experimental Pharmacology, 210, 3–22. doi:10.1007/978-3-642-29423-5_1.
van Nispen tot Pannerden, H., de Haas, F., Geerts, W., Posthuma, G., van Dijk, S., & Heijnen, H. F. (2010). The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood, 116(7), 1147–1156. doi:10.1182/blood-2010-02-268680.
Choi, W., Karim, Z. A., & Whiteheart, S. W. (2010). Protein expression in platelets from six species that differ in their open canalicular system. Platelets, 21(3), 167–175. doi:10.3109/09537101003611385.
Hughes, F. B., & Brodie, B. B. (1959). The mechanism of serotonin and catecholamine uptake by platelets. The Journal of Pharmacology and Experimental Therapeutics, 127, 96–102.
Adnot, S., Houssaini, A., Abid, S., Marcos, E., & Amsellem, V. (2013). Serotonin transporter and serotonin receptors. Handbook of Experimental Pharmacology, 218, 365–380. doi:10.1007/978-3-642-38664-0_15.
Pavanetto, M., Zarpellon, A., Borgo, C., Donella-Deana, A., & Deana, R. (2011). Regulation of serotonin transport in human platelets by tyrosine kinase Syk. Cellular Physiology and Biochemistry, 27(2), 139–148. doi:10.1159/000325216.
Jin, K., Li, T., van Dam, H., Zhou, F., & Zhang, L. (2017). Molecular insights into tumour metastasis: tracing the dominant events. The Journal of Pathology, 241(5), 567–577. doi:10.1002/path.4871.
Mitrugno, A., Tormoen, G. W., Kuhn, P., & McCarty, O. J. (2016). The prothrombotic activity of cancer cells in the circulation. Blood Reviews, 30(1), 11–19. doi:10.1016/j.blre.2015.07.001.
Hisada, Y., Geddings, J. E., Boulaftali, Y., Getz, T. M., Whelihan, M., Fuentes, R., et al. (2016). OC-04—tissue factor positive microvesicles activate platelets in vitro and in vivo and enhance thrombosis in mice. Thrombosis Research, 140(Suppl 1), S169–S170. doi:10.1016/S0049-3848(16)30121-9.
Geddings, J. E., Hisada, Y., Boulaftali, Y., Getz, T. M., Whelihan, M., Fuentes, R., et al. (2016). Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. Journal of Thrombosis and Haemostasis, 14(1), 153–166. doi:10.1111/jth.13181.
Tseng, J. C., Chang, L. C., Jiang, B. Y., Liu, Y. C., Chen, H. J., Yu, C. T., et al. (2014). Elevated circulating levels of tissue factor-positive microvesicles are associated with distant metastasis in lung cancer. Journal of Cancer Research and Clinical Oncology, 140(1), 61–67. doi:10.1007/s00432-013-1544-8.
Heijnen, H., & van der Sluijs, P. (2015). Platelet secretory behaviour: as diverse as the granules ... or not? Journal of Thrombosis and Haemostasis, 13(12), 2141–2151. doi:10.1111/jth.13147.
Chen, C. H., Lo, R. W., Urban, D., Pluthero, F. G., & Kahr, W. H. (2017). Alpha-granule biogenesis: from disease to discovery. Platelets, 28(2), 147–154. doi:10.1080/09537104.2017.1280599.
White, J. G. (1972). Exocytosis of secretory organelles from blood platelets incubated with cationic polypeptides. The American Journal of Pathology, 69(1), 41–54.
White, J. G., & Estensen, R. D. (1972). Degranulation of discoid platelets. The American Journal of Pathology, 68(2), 289–302.
Harrison, P., & Cramer, E. M. (1993). Platelet alpha-granules. Blood Reviews, 7(1), 52–62.
Yadav, S., & Storrie, B. (2017). The cellular basis of platelet secretion: emerging structure/function relationships. Platelets, 28(2), 108–118. doi:10.1080/09537104.2016.1257786.
Sander, H. J., Slot, J. W., Bouma, B. N., Bolhuis, P. A., Pepper, D. S., & Sixma, J. J. (1983). Immunocytochemical localization of fibrinogen, platelet factor 4, and beta thromboglobulin in thin frozen sections of human blood platelets. The Journal of Clinical Investigation, 72(4), 1277–1287. doi:10.1172/jci111084.
Wencel-Drake, J. D., Painter, R. G., Zimmerman, T. S., & Ginsberg, M. H. (1985). Ultrastructural localization of human platelet thrombospondin, fibrinogen, fibronectin, and von Willebrand factor in frozen thin section. Blood, 65(4), 929–938.
White, J. G. (1968). The dense bodies of human platelets. Origin of serotonin storage particles from platelet granules. The American Journal of Pathology, 53(5), 791–808.
Flaumenhaft, R. (2006). Formation and fate of platelet microparticles. Blood Cells, Molecules & Diseases, 36(2), 182–187. doi:10.1016/j.bcmd.2005.12.019.
Horstman, L. L., & Ahn, Y. S. (1999). Platelet microparticles: a wide-angle perspective. Critical Reviews in Oncology/Hematology, 30(2), 111–142.
Wolf, P. (1967). The nature and significance of platelet products in human plasma. British Journal of Haematology, 13(3), 269–288.
Heijnen, H. F., Schiel, A. E., Fijnheer, R., Geuze, H. J., & Sixma, J. J. (1999). Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood, 94(11), 3791–3799.
Zmigrodzka, M., Guzera, M., Miskiewicz, A., Jagielski, D., & Winnicka, A. (2016). The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumour Biology, 37(11), 14391–14401. doi:10.1007/s13277-016-5358-6.
Janowska-Wieczorek, A., Wysoczynski, M., Kijowski, J., Marquez-Curtis, L., Machalinski, B., Ratajczak, J., et al. (2005). Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. International Journal of Cancer, 113(5), 752–760. doi:10.1002/ijc.20657.
Hugel, B., Martinez, M. C., Kunzelmann, C., & Freyssinet, J. M. (2005). Membrane microparticles: two sides of the coin. Physiology (Bethesda), 20, 22–27. doi:10.1152/physiol.00029.2004.
Bobrie, A., Colombo, M., Raposo, G., & Thery, C. (2011). Exosome secretion: molecular mechanisms and roles in immune responses. Traffic, 12(12), 1659–1668. doi:10.1111/j.1600-0854.2011.01225.x.
Varon, D., & Shai, E. (2009). Role of platelet-derived microparticles in angiogenesis and tumor progression. Discovery Medicine, 8(43), 237–241.
Dovizio, M., Alberti, S., Guillem-Llobat, P., & Patrignani, P. (2014). Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic & Clinical Pharmacology & Toxicology, 114(1), 118–127. doi:10.1111/bcpt.12156.
Mause, S. F., & Weber, C. (2010). Microparticles: protagonists of a novel communication network for intercellular information exchange. Circulation Research, 107(9), 1047–1057. doi:10.1161/circresaha.110.226456.
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., & Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659. doi:10.1038/ncb1596.
Weidle, U. H., Birzele, F., Kollmorgen, G., & Ruger, R. (2017). The multiple roles of exosomes in metastasis. Cancer Genomics Proteomics, 14(1), 1–15. doi:10.21873/cgp.20015.
Chaput, N., & Thery, C. (2011). Exosomes: immune properties and potential clinical implementations. Seminars in Immunopathology, 33(5), 419–440. doi:10.1007/s00281-010-0233-9.
Thery, C., Zitvogel, L., & Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nature Reviews. Immunology, 2(8), 569–579. doi:10.1038/nri855.
Thery, C., Boussac, M., Veron, P., Ricciardi-Castagnoli, P., Raposo, G., Garin, J., et al. (2001). Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. Journal of Immunology, 166(12), 7309–7318.
Honn, K. V., Tang, D. G., & Chen, Y. Q. (1992). Platelets and cancer metastasis: more than an epiphenomenon. Seminars in Thrombosis and Hemostasis, 18(4), 392–415. doi:10.1055/s-2007-1002578.
Zucker, S., Pei, D., Cao, J., & Lopez-Otin, C. (2003). Membrane type-matrix metalloproteinases (MT-MMP). Current Topics in Developmental Biology, 54, 1–74.
Jansen, F., Yang, X., Hoyer, F. F., Paul, K., Heiermann, N., Becher, M. U., et al. (2012). Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(8), 1925–1935. doi:10.1161/atvbaha.112.253229.
Ciravolo, V., Huber, V., Ghedini, G. C., Venturelli, E., Bianchi, F., Campiglio, M., et al. (2012). Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. Journal of Cellular Physiology, 227(2), 658–667. doi:10.1002/jcp.22773.
Helley, D., Banu, E., Bouziane, A., Banu, A., Scotte, F., Fischer, A. M., et al. (2009). Platelet microparticles: a potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. European Urology, 56(3), 479–484. doi:10.1016/j.eururo.2008.06.038.
Kim, H. K., Song, K. S., Park, Y. S., Kang, Y. H., Lee, Y. J., Lee, K. R., et al. (2003). Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. European Journal of Cancer, 39(2), 184–191.
Italiano Jr., J. E., Mairuhu, A. T., & Flaumenhaft, R. (2010). Clinical relevance of microparticles from platelets and megakaryocytes. Current Opinion in Hematology, 17(6), 578–584. doi:10.1097/MOH.0b013e32833e77ee.
Kral, J. B., Schrottmaier, W. C., Salzmann, M., & Assinger, A. (2016). Platelet interaction with innate immune cells. Transfusion Medicine and Hemotherapy, 43(2), 78–88. doi:10.1159/000444807.
Manne, B. K., Xiang, S. C., & Rondina, M. T. (2017). Platelet secretion in inflammatory and infectious diseases. Platelets, 28(2), 155–164. doi:10.1080/09537104.2016.1240766.
Thomas, M. R., & Storey, R. F. (2015). The role of platelets in inflammation. Thrombosis and Haemostasis, 114(3), 449–458. doi:10.1160/TH14-12-1067.
Laurance, S., Bertin, F. R., Ebrahimian, T., Kassim, Y., Rys, R. N., Lehoux, S., et al. (2017). Gas6 (growth arrest-specific 6) promotes inflammatory (CCR2hiCX3CR1lo) monocyte recruitment in venous thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. doi:10.1161/ATVBAHA.116.308925.
Frydman, G. H., Le, A., Ellett, F., Jorgensen, J., Fox, J. G., Tompkins, R. G., et al. (2017). Technical advance: changes in neutrophil migration patterns upon contact with platelets in a microfluidic assay. Journal of Leukocyte Biology, 101(3), 797–806. doi:10.1189/jlb.1TA1115-517RR.
Zuchtriegel, G., Uhl, B., Puhr-Westerheide, D., Pornbacher, M., Lauber, K., Krombach, F., et al. (2016). Platelets guide leukocytes to their sites of extravasation. PLoS Biology, 14(5), e1002459. doi:10.1371/journal.pbio.1002459.
Yan, M., & Jurasz, P. (2016). The role of platelets in the tumor microenvironment: from solid tumors to leukemia. Biochimica et Biophysica Acta, 1863(3), 392–400. doi:10.1016/j.bbamcr.2015.07.008.
Kim, J., & Bae, J. S. (2016). Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators of Inflammation, 2016, 6058147. doi:10.1155/2016/6058147.
Rossaint, J., & Zarbock, A. (2015). Platelets in leucocyte recruitment and function. Cardiovascular Research, 107(3), 386–395. doi:10.1093/cvr/cvv048.
Dohlman, T. H., Di Zazzo, A., Omoto, M., Hua, J., Ding, J., Hamrah, P., et al. (2016). E-selectin mediates immune cell trafficking in corneal transplantation. Transplantation, 100(4), 772–780. doi:10.1097/TP.0000000000001107.
Dinkla, S., van Cranenbroek, B., van der Heijden, W. A., He, X., Wallbrecher, R., Dumitriu, I. E., et al. (2016). Platelet microparticles inhibit IL-17 production by regulatory T cells through P-selectin. Blood, 127(16), 1976–1986. doi:10.1182/blood-2015-04-640300.
Scotland, R. S., Cohen, M., Foster, P., Lovell, M., Mathur, A., Ahluwalia, A., et al. (2005). C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proceedings of the National Academy of Sciences of the United States of America, 102(40), 14452–14457. doi:10.1073/pnas.0504961102.
Diacovo, T. G., Roth, S. J., Morita, C. T., Rosat, J. P., Brenner, M. B., & Springer, T. A. (1996). Interactions of human alpha/beta and gamma/delta T lymphocyte subsets in shear flow with E-selectin and P-selectin. The Journal of Experimental Medicine, 183(3), 1193–1203.
Hu, M., Zhang, H., Liu, Q., & Hao, Q. (2016). Structural basis for human PECAM-1-mediated trans-homophilic cell adhesion. Scientific Reports, 6, 38655. doi:10.1038/srep38655.
Vachino, G., Chang, X. J., Veldman, G. M., Kumar, R., Sako, D., Fouser, L. A., et al. (1995). P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. The Journal of Biological Chemistry, 270(37), 21966–21974.
Duchez, A. C., Boudreau, L. H., Naika, G. S., Bollinger, J., Belleannee, C., Cloutier, N., et al. (2015). Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proceedings of the National Academy of Sciences of the United States of America, 112(27), E3564–E3573. doi:10.1073/pnas.1507905112.
Gay, L. J., & Felding-Habermann, B. (2011). Contribution of platelets to tumour metastasis. Nature Reviews. Cancer, 11(2), 123–134. doi:10.1038/nrc3004.
Palumbo, J. S., Talmage, K. E., Massari, J. V., La Jeunesse, C. M., Flick, M. J., Kombrinck, K. W., et al. (2005). Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood, 105(1), 178–185. doi:10.1182/blood-2004-06-2272.
Calverley, D. C., Phang, T. L., Choudhury, Q. G., Gao, B., Oton, A. B., Weyant, M. J., et al. (2010). Significant downregulation of platelet gene expression in metastatic lung cancer. Clinical and Translational Science, 3(5), 227–232.
Nilsson, R. J., Balaj, L., Hulleman, E., van Rijn, S., Pegtel, D. M., Walraven, M., et al. (2011). Blood platelets contain tumor-derived RNA biomarkers. Blood, 118(13), 3680–3683. doi:10.1182/blood-2011-03-344408.
Joosse, S. A., & Pantel, K. (2015). Tumor-educated platelets as liquid biopsy in cancer patients. Cancer Cell, 28(5), 552–554. doi:10.1016/j.ccell.2015.10.007.
Best, M. G., Sol, N., Kooi, I., Tannous, J., Westerman, B. A., Rustenburg, F., et al. (2015). RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell, 28(5), 666–676. doi:10.1016/j.ccell.2015.09.018.
Zhu, J., & Strickler, J. H. (2016). Clinical applications of liquid biopsies in gastrointestinal oncology. J Gastrointest Oncol, 7(5), 675–686. doi:10.21037/jgo.2016.08.08.
Feller, S. M., & Lewitzky, M. (2016). Hunting for the ultimate liquid cancer biopsy—let the TEP dance begin. Cell Communication and Signaling: CCS, 14(1), 24. doi:10.1186/s12964-016-0147-9.
Holmes, C. E., Levis, J. E., Schneider, D. J., Bambace, N. M., Sharma, D., Lal, I., et al. (2016). Platelet phenotype changes associated with breast cancer and its treatment. Platelets, 27(7), 703–711. doi:10.3109/09537104.2016.1171302.
Dixon, D. A., Tolley, N. D., Bemis-Standoli, K., Martinez, M. L., Weyrich, A. S., Morrow, J. D., et al. (2006). Expression of COX-2 in platelet-monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. The Journal of Clinical Investigation, 116(10), 2727–2738. doi:10.1172/jci27209.
Evangelista, V., Manarini, S., Di Santo, A., Capone, M. L., Ricciotti, E., Di Francesco, L., et al. (2006). De novo synthesis of cyclooxygenase-1 counteracts the suppression of platelet thromboxane biosynthesis by aspirin. Circulation Research, 98(5), 593–595. doi:10.1161/01.RES.0000214553.37930.3e.
Guillem-Llobat, P., Dovizio, M., Alberti, S., Bruno, A., & Patrignani, P. (2014). Platelets, cyclooxygenases, and colon cancer. Seminars in Oncology, 41(3), 385–396. doi:10.1053/j.seminoncol.2014.04.008.
Mitrugno, A., Sylman, J. L., Ngo, A. T., Pang, J., Sears, R. C., Williams, C. D., et al. (2017). Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: implications for the oncoprotein c-MYC. American Journal of Physiology. Cell Physiology, 312(2), C176–c189. doi:10.1152/ajpcell.00196.2016.
Radziwon-Balicka, A., Santos-Martinez, M. J., Corbalan, J. J., O'Sullivan, S., Treumann, A., Gilmer, J. F., et al. (2014). Mechanisms of platelet-stimulated colon cancer invasion: role of clusterin and thrombospondin 1 in regulation of the P38MAPK-MMP-9 pathway. Carcinogenesis, 35(2), 324–332. doi:10.1093/carcin/bgt332.
Labelle, M., Begum, S., & Hynes, R. O. (2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell, 20(5), 576–590. doi:10.1016/j.ccr.2011.09.009.
Cooke, N. M., Spillane, C. D., Sheils, O., O'Leary, J., & Kenny, D. (2015). Aspirin and P2Y12 inhibition attenuate platelet-induced ovarian cancer cell invasion. BMC Cancer, 15, 627. doi:10.1186/s12885-015-1634-x.
Mammadova-Bach, E., Zigrino, P., Brucker, C., Bourdon, C., Freund, M., De Arcangelis, A., et al. (2016). Platelet integrin alpha6beta1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI Insight, 1(14), e88245. doi:10.1172/jci.insight.88245.
Menter, D. G., Onoda, J. M., Taylor, J. D., & Honn, K. V. (1984). Effects of prostacyclin on tumor cell-induced platelet aggregation. Cancer Research, 44(2), 450–456.
Menter, D. G., Harkins, C., Onoda, J., Riorden, W., Sloane, B. F., Taylor, J. D., et al. (1987). Inhibition of tumor cell induced platelet aggregation by prostacyclin and carbacyclin: an ultrastructural study. Invasion & Metastasis, 7(2), 109–128.
Gasic, G. J., & Gasic, T. B. (1982). Plasma membrane vesicles as mediators of interactions between tumor cells and components of the hemostatic and immune systems. Progress in Clinical and Biological Research, 89, 429–444.
Gasic, G. J., Tuszynski, G. P., & Gorelik, E. (1986). Interaction of the hemostatic and immune systems in the metastatic spread of tumor cells. International Review of Experimental Pathology, 29, 173–212.
Ruppert, M., Aigner, S., Hubbe, M., Yagita, H., & Altevogt, P. (1995). The L1 adhesion molecule is a cellular ligand for VLA-5. The Journal of Cell Biology, 131(6 Pt 2), 1881–1891.
Tsuruo, T., & Fujita, N. (2008). Platelet aggregation in the formation of tumor metastasis. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 84(6), 189–198.
Menter, D. G., Steinert, B. W., Sloane, B. F., Taylor, J. D., & Honn, K. V. (1987). A new in vitro model for investigation of tumor cell-platelet-endothelial cell interactions and concomitant eicosanoid biosynthesis. Cancer Research, 47(9), 2425–2432.
Menter, D. G., Onoda, J. M., Moilanen, D., Sloane, B. F., Taylor, J. D., & Honn, K. V. (1987). Inhibition by prostacyclin of the tumor cell-induced platelet release reaction and platelet aggregation. Journal of the National Cancer Institute, 78(5), 961–969.
Xu, L., Mao, X., Imrali, A., Syed, F., Mutsvangwa, K., Berney, D., et al. (2015). Optimization and evaluation of a novel size based circulating tumor cell isolation system. PloS One, 10(9), e0138032. doi:10.1371/journal.pone.0138032.
Sorensen, H. T., Mellemkjaer, L., Steffensen, F. H., Olsen, J. H., & Nielsen, G. L. (1998). The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. The New England Journal of Medicine, 338(17), 1169–1173. doi:10.1056/nejm199804233381701.
Levitan, N., Dowlati, A., Remick, S. C., Tahsildar, H. I., Sivinski, L. D., Beyth, R., et al. (1999). Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore), 78(5), 285–291.
Alexandrakis, M. G., Passam, F. H., Moschandrea, I. A., Christophoridou, A. V., Pappa, C. A., Coulocheri, S. A., et al. (2003). Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. American Journal of Clinical Oncology, 26(2), 135–140. doi:10.1097/01.coc.0000017093.79897.de.
Seretis, C., Youssef, H., & Chapman, M. (2015). Hypercoagulation in colorectal cancer: what can platelet indices tell us? Platelets, 26(2), 114–118. doi:10.3109/09537104.2014.894969.
Karagöz, B., Sücüllü, İ., Sayan, Ö., Bilgi, O., Tuncel, T., Filiz, A. İ., et al. (2010). Platelet indices in patients with colorectal cancer. [journal article]. Central European Journal of Medicine, 5(3), 365–368. doi:10.2478/s11536-009-0077-7.
Watt, D. G., Proctor, M. J., Park, J. H., Horgan, P. G., & McMillan, D. C. (2015). The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PloS One, 10(11), e0142159. doi:10.1371/journal.pone.0142159.
Wan, S., Lai, Y., Myers, R. E., Li, B., Hyslop, T., London, J., et al. (2013). Preoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patients. Journal of Gastrointestinal Cancer, 44(3), 293–304. doi:10.1007/s12029-013-9491-9.
Zhao, J. M., Wang, Y. H., Yao, N., Wei, K. K., Jiang, L., Hanif, S., et al. (2016). Poor prognosis significance of pretreatment thrombocytosis in patients with colorectal cancer: a meta-analysis. Asian Pacific Journal of Cancer Prevention, 17(9), 4295–4300.
Wodarczyk, M., Kasprzyk, J., Sobolewska-Wodarczyk, A., Wodarczyk, J., Tchorzewski, M., Dziki, A., et al. (2016). Mean platelet volume as a possible biomarker of tumor progression in rectal cancer. Cancer Biomarkers, 17(4), 411–417. doi:10.3233/cbm-160657.
Dymicka-Piekarska, V., Matowicka-Karna, J., Osada, J., Kemona, H., & Butkiewicz, A. M. (2006). Changes in platelet CD 62P expression and soluble P-selectin concentration in surgically treated colorectal carcinoma. Advances in Medical Sciences, 51, 304–308.
Dymicka-Piekarska, V., Kemona, H., Piotrowski, Z., Gryko, M., Milewski, Z., & Matowicka-Karna, J. (2003). Does colorectal cancer influence platelet activation? Przegla̧d Lekarski, 60(11), 716–718.
Del Rio, M., Mollevi, C., Vezzio-Vie, N., Bibeau, F., Ychou, M., & Martineau, P. (2013). Specific extracellular matrix remodeling signature of colon hepatic metastases. PloS One, 8(9), e74599. doi:10.1371/journal.pone.0074599.
Zhao, L., Bi, Y., Kou, J., Shi, J., & Piao, D. (2016). Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. Journal of Experimental & Clinical Cancer Research, 35, 54. doi:10.1186/s13046-016-0328-9.
Mantur, M., Snarska, J., Sidorska, A., Ostrowska, H., Kruszewska-Wnorowska, K., & Wojszel, J. (2008). Changes in PDGF concentration in surgically treated colorectal carcinoma. Advances in Medical Sciences, 53(1), 37–41. doi:10.2478/v10039-008-0030-z.
Ogino, S., Kirkner, G. J., Nosho, K., Irahara, N., Kure, S., Shima, K., et al. (2008). Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clinical Cancer Research, 14(24), 8221–8227. doi:10.1158/1078-0432.ccr-08-1841.
Sharma, D., Brummel-Ziedins, K. E., Bouchard, B. A., & Holmes, C. E. (2014). Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. Journal of Cellular Physiology, 229(8), 1005–1015. doi:10.1002/jcp.24539.
Sostres, C., Gargallo, C. J., & Lanas, A. (2014). Aspirin, cyclooxygenase inhibition and colorectal cancer. World J Gastrointest Pharmacol Ther, 5(1), 40–49. doi:10.4292/wjgpt.v5.i1.40.
Su, B. B., Chen, J. H., Shi, H., Chen, Q. Q., & Wan, J. (2014). Aspirin may modify tumor microenvironment via antiplatelet effect. Medical Hypotheses, 83(2), 148–150. doi:10.1016/j.mehy.2014.05.007.
Guillem-Llobat, P., Dovizio, M., Bruno, A., Ricciotti, E., Cufino, V., Sacco, A., et al. (2016). Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget, 7(22), 32462–32477. doi:10.18632/oncotarget.8655.
Roop, R. P., Naughton, M. J., Van Poznak, C., Schneider, J. G., Lammers, P. E., Pluard, T. J., et al. (2013). A randomized phase II trial investigating the effect of platelet function inhibition on circulating tumor cells in patients with metastatic breast cancer. Clinical Breast Cancer, 13(6), 409–415. doi:10.1016/j.clbc.2013.08.006.
Holmes, C. E., Jasielec, J., Levis, J. E., Skelly, J., & Muss, H. B. (2013). Initiation of aspirin therapy modulates angiogenic protein levels in women with breast cancer receiving tamoxifen therapy. Clinical and Translational Science, 6(5), 386–390. doi:10.1111/cts.12070.
Thun, M. J., Henley, S. J., & Patrono, C. (2002). Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. Journal of the National Cancer Institute, 94(4), 252–266.
Alonso-Escolano, D., Strongin, A. Y., Chung, A. W., Deryugina, E. I., & Radomski, M. W. (2004). Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. British Journal of Pharmacology, 141(2), 241–252. doi:10.1038/sj.bjp.0705606.
Frouws, M. A., Rademaker, E., Bastiaannet, E., van Herk-Sukel, M. P., Lemmens, V. E., Van de Velde, C. J., et al. (2017). The difference in association between aspirin use and other thrombocyte aggregation inhibitors and survival in patients with colorectal cancer. European Journal of Cancer, 77, 24–30. doi:10.1016/j.ejca.2017.02.025.
Liang, H., Yang, C., Zhang, B., Wang, H., Liu, H., Zhao, Z., et al. (2015). Hydroxyethyl starch 200/0.5 decreases circulating tumor cells of colorectal cancer patients and reduces metastatic potential of colon cancer cell line through inhibiting platelets activation. Medical Oncology, 32(5), 151. doi:10.1007/s12032-015-0601-3.
Zhang, Y., Wei, J., Liu, S., Wang, J., Han, X., Qin, H., et al. (2017). Inhibition of platelet function using liposomal nanoparticles blocks tumor metastasis. Theranostics, 7(5), 1062–1071. doi:10.7150/thno.17908.
Ludwig, R. J., Schon, M. P., & Boehncke, W. H. (2007). P-selectin: a common therapeutic target for cardiovascular disorders, inflammation and tumour metastasis. Expert Opinion on Therapeutic Targets, 11(8), 1103–1117. doi:10.1517/14728222.11.8.1103.
Qi, C., Li, B., Guo, S., Wei, B., Shao, C., Li, J., et al. (2015). P-selectin-mediated adhesion between platelets and tumor cells promotes intestinal tumorigenesis in Apc(min/+) mice. International Journal of Biological Sciences, 11(6), 679–687. doi:10.7150/ijbs.11589.
Dymicka-Piekarska, V., Butkiewicz, A., Matowicka-Karna, J., & Kemona, H. (2005). Soluble P-selectin concentration in patients with colorectal cancer. Neoplasma, 52(4), 297–301.
Dymicka-Piekarska, V., Matowicka-Karna, J., Gryko, M., Kemona-Chetnik, I., & Kemona, H. (2007). Relationship between soluble P-selectin and inflammatory factors (interleukin-6 and C-reactive protein) in colorectal cancer. Thrombosis Research, 120(4), 585–590. doi:10.1016/j.thromres.2006.11.002.
Li, J., Sharkey, C. C., Wun, B., Liesveld, J. L., & King, M. R. (2016). Genetic engineering of platelets to neutralize circulating tumor cells. Journal of Controlled Release, 228, 38–47. doi:10.1016/j.jconrel.2016.02.036.
Acknowledgements
Grant and other support: Boone Pickens Distinguished Chair for Early Prevention of Cancer, Duncan Family Institute, Colorectal Cancer Moon Shot, P30CA016672-41, 1R01CA187238-01, 5R01CA172670-03 and 1R01CA184843-01A1, and CA177909.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kanikarla-Marie, P., Lam, M., Menter, D.G. et al. Platelets, circulating tumor cells, and the circulome. Cancer Metastasis Rev 36, 235–248 (2017). https://doi.org/10.1007/s10555-017-9681-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-017-9681-1