Abstract
In patients with repaired tetralogy of Fallot (rTOF), the regurgitant fraction (RF) in left pulmonary artery (LPA) and right pulmonary artery (RPA) is usually unequal. The morphometrics may play a crucial role in this RF discrepancy. Cardiovascular MR of 79 rTOF patients and 20 healthy controls were retrospectively enrolled. Forty-four from the 79 patients were matched in age, sex and body surface area to the 20 controls and were investigated for: (1) phase-contrast flow of main pulmonary artery (MPA), LPA, and RPA; (2) vascular angles: the angles between the thoracic anterior–posterior line (TAPL) with MPA (θM–AP), MPA with RPA (θM–R), and MPA with LPA (θM–L); (3) cardiac angle, the angle between TAPL and the interventricular septum; (4) area ratio of bilateral lung and hemithorax regions. Compared with the 20 controls, the 44 rTOF patients exhibited wider θM–AP, sharper θM–L angle, and a smaller θM–L/θM–R ratio. In the 79 rTOF patients, LPA showed lower forward, backward, and net flow, and greater RF as compared with RPA. Multivariate analysis showed that the RF of LPA was negatively associated with the θM–L/θM–R ratio and the age at surgery (R2 = 0.255). Conversely, the RF of RPA was negatively associated with the left lung/left hemithorax area ratio and cross-sectional area (CSA) of LPA, and positively associated with CSA of RPA and MPA (R2 = 0.366). In rTOF patients, the RF of LPA is more severe than that of RPA, which may be related to the vascular morphometrics. Different morphometric parameters are independently associated with the RF of LPA or RPA, which may offer potential insights for surgical strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary regurgitation (PR) is the most important complication in patients with repaired tetralogy of Fallot (rTOF). Although variable degrees of PR related to rTOF can be tolerated for many years, PR in some rTOF patients becomes severe and is associated with dilatation and dysfunction of the right ventricle (RV), and subsequently the left ventricle, therefore contributing to long-term morbidities, including exercise intolerance, atrial and ventricular arrhythmia, congestive heart failure, and possibly, sudden death [1, 2].
Cardiovascular MR (CMR) is regarded as part of standard care in serial follow-up studies of rTOF patients [3]. Phase-contrast magnetic resonance imaging (PC-MRI) is accepted as the imaging modality of choice for the quantification of PR due to its accurate and reproducible measurement of the flow volume, velocity, and blood flow patterns [4], even in patients with suspected peripheral pulmonary stenosis [5]. By using the contrast-enhanced magnetic resonance angiography (CE-MRA) with 3-dimensional (3D) reconstruction, the morphometrics of the pulmonary arteries and cardiopulmonary anatomy can be well assessed and analyzed.
Previous studies have shown that the branch pulmonary arteries in rTOF patients often have differential RF and contribute unequally to total PR [6,7,8]. The RF of LPA is usually larger than that of RPA. The cause of differential RF is still unclear. Kato et al. [9] discovered that an enlarged and levorotated heart is related to compression of the left lung, which in turn contributes to increased diastolic flow reversal in LPA. Chern et al. [10] found that in rTOF patients, the regurgitation initially occurs in LPA because of the small angle between LPA and MPA, and a recirculation zone impeding the forward pulmonary blood flow is observed within LPA during the acceleration of systole. However, no large-scale studies have been reported to comprehensively evaluate the contributory factors of differential branch RF in rTOF patients.
We hypothesized that the vascular morphometrics may play an important role in the mechanism of differential RF in bilateral pulmonary arteries of rTOF patients. In this retrospective CMR study, we compared the morphometrics between rTOF patients and the normal controls. We anticipated that the results may provide morphophysiological insights that may be useful for surgical consideration in the management of TOF or rTOF patients.
Materials and methods
Patients
Between January 2004 and October 2018, patients who underwent CMR surveillance after total repair of tetralogy of Fallot at Kaohsiung Veterans General Hospital were retrospectively reviewed. There were 118 cases identified within the Research Information System. Among them, thirteen cases exhibited incomplete CMR image sequences or suboptimal image quality for evaluation. A total of 26 patients were excluded due to the following causes: rTOF with pulmonary valve replacement (n = 4), post-endovascular stent placement in pulmonary arteries (n = 2), significant branch pulmonary artery stenosis (defined as > 50% reduction in diameter compared to the proximal abutting vessel [11], n = 8) and incomplete surgical procedure records (n = 12). As a result, 79 rTOF patients were enrolled in this study. Patient characteristics were shown as Table 1.
We also reviewed our previous CMR case collections of healthy volunteers for use as a normal control group. The inclusion criteria for this group were: 1. Age and sex similar to that of the patient group; 2. Absence of symptoms and signs or established diagnosis of cardiopulmonary diseases during the initial scan and subsequently reconfirmed. As a result, a normal control group of 20 healthy individuals was retrospectively enrolled in this study. However, there was a difference of age, sex and body surface area (BSA) between the two groups. To further remove these potential confounding factors, we then selected 44 from the 79 rTOF patients with matched age, sex and BSA to the 20 controls for comparison. As a result, there was no statistically significant difference of age, sex and BSA between the two groups.
The Research Ethics Board of our institute approved this study. All patients or patients' parents, depending on the patients’ age, provided written informed consents.
MRI protocol
CMR studies were performed on a 1.5-T system (Signa CV/I, GE Healthcare, Milwaukee, Wisconsin). PC-MRI acquisition was applied for MPA, RPA, and LPA. The effective temporal resolution was regulated to allow for 20 reconstructed phases per cardiac cycle. Besides, CSA of the pulmonary arteries were generated from the selected contours encircling MPA, RPA, and LPA on the magnitude images of the phase contrast acquisition. Acquired PC-MRI data were processed in an independent workstation. The flow analysis software (CVI42, Circle Cardiovascular Imaging INC., Calgary, Canada) was used to generate flow volume-time curves of the MPA, RPA, and LPA. The flow measurement included forward flow volume (FFV), backward flow volume (BFV), net flow volume (NFV), and RF. NFV was defined as FFV minus BFV. RF was defined as BFV divided by FFV.
Morphometric definitions
Source images of 3D CE-MRA images were reconstructed on the workstation (AW server 3.2 Ext. 4.0, GE Healthcare, Milwaukee, Wisconsin). Images were reconstructed in three standardized projections as shown in Fig. 1 for the measurement of pulmonary artery angles. We measured the angle between the thoracic anterior–posterior line and MPA (defined as θM–AP, Fig. 1a), between the thoracic anterior–posterior line and LPA (defined as θL–AP, Fig. 1b), and between the thoracic anterior–posterior line and RPA (defined as θR–AP, Fig. 1c) on axial images. MPA, RPA, and LPA were identified individually because they may not lie in the same axial plane. The angle between the MPA and RPA (defined as θM–R, Fig. 1d) can be calculated as follows: θM–R = 180—θR–AP + θM–AP. The angle between the MPA and LPA (defined asθM–L, Fig. 1d) can be calculated as follows: θM–L = 180—θM–AP–θL–AP.
Axial plane images for measuring pulmonary artery angles a The angle between the thoracic anterior–posterior line and main pulmonary artery was measured, defined as θM–AP. b The angle between the thoracic anterior–posterior line and left pulmonary artery was measured, defined as θL–AP. c The angle between the thoracic anterior–posterior line and right pulmonary artery was measured, defined as θR–AP. d The angle between main pulmonary artery and right pulmonary artery (defined as θM–R) can be calculated as follows: θM–R = 180—θR–AP + θM–AP. The angle between main pulmonary artery and left pulmonary artery (defined as θM–L) can be calculated as follows: θM–L = 180—θM–AP—θL–AP AP anterior–posterior, LPA left pulmonary artery, MPA main pulmonary artery, RPA right pulmonary artery
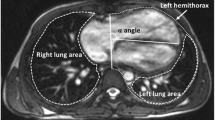
According to the report of Kato et al. [9] (Fig. 2), we measured the cardiac angle (referred to as α angle) and calculated the lung area ratio. Measurements were taken on the axial plane image displaying the largest cardiac surface area. The cardiac angle was defined as the angle between the thoracic anterior–posterior line and the interventricular septum. Lung area ratio was calculated as the quotient of lung area to the ipsilateral hemithorax area ratio.
Statistical analysis
The parameters were expressed as median (interquartile range) or mean ± standard deviation according to normality tests and analyzed by paired samples t-test. Correlation between parameters was evaluated by Pearson correlation analysis. Because the RF of LPA, RPA, and MPA were normally distributed, multivariate linear regression was used for predicting the effect of morphological and clinical profiles on the RF. All p < 0.05, 2-sided, were considered statistically significant. All analyses were done with IBM SPSS Statistics, version 22.0.
Results
PC-MRI flow measurement
The flow volumes of the LPA are significantly lower than those of RPA in terms of FFV, BFV, and NFV, while the CSA are similar between LPA and RPA. In contrary, the RF of LPA was 0.42, which was higher than the RF of RPA (0.30) (p < 0.001). The details are listed in Table 2.
Pulmonary artery angle
For the matched 44 rTOF patients to the normal control group, θM–AP and θM–R were larger in matched rTOF patients than in the normal control group (p < 0.001), whereas θM–L was smaller (p < 0.001). As a result, the θM–L/θM–R ratio was significantly smaller in the matched rTOF patients group than in the normal control group (p < 0.001) (Table 3).
The relationship between multiple variables and regurgitant fraction
The relationships between time interval factors, flow factors, morphometric factors, and RF are shown in Table 4. The RF of LPA, the RF of RPA, and the RF of MPA correlated with each other significantly. Left lung area ratio correlated inversely with the RF of LPA (Fig. 3a) and α angle, the same result as the report of Kato et al. [9]. Left lung ratio also correlated inversely with the RF of RPA (Fig. 3b) and the RF of MPA (Fig. 3c). The RF of LPA did not correlate with CSA of LPA (Fig. 4a), whereas the RF of RPA correlated with CSA of RPA (Fig. 4b), and the RF of MPA correlated with CSA of MPA (Fig. 4c). The RF of LPA correlated inversely with θM–L/θM–R ratio (Fig. 5a) and age at surgery, and the RF of RPA also correlated inversely with θM–L/θM–R ratio (Fig. 5b).
The multivariate linear regression analysis involved the creation of two distinct models. Model 1 encompassed all factors, including the MPA flow parameters, whereas model 2 excluded the MPA flow parameters from the independent variables. For predictors of the RF of LPA, the model 1 (Table 4) included CSA of MPA, θM–L/θM–R ratio, age at surgery, and BFV of MPA (R2 = 0.511); the model 2 included θM–L/θM–R ratio and age at surgery (R2 = 0.255). For predictors of the RF of RPA, the model 1 included the RF of MPA, left lung area ratio, CSA of LPA, and CSA of RPA (R2 = 0.613); the model 2 included left lung area ratio, CSA of LPA, CSA of RPA, and CSA of MPA (R2 = 0.366). In addition, the predictors of the RF of MPA include left lung area ratio and CSA of MPA (R2 = 0.223).
Discussion
In this study, we investigated the morphology of pulmonary arteries and their relationship with RF in branch pulmonary arteries of rTOF patients by CMR. The main findings of our study can be summarized as follows: (1) The morphometrics of rTOF patients is characteristically different from that of the normal control group: (1a) The course of MPA in rTOF patients tilted more to the left side. (1b) rTOF patients had larger θM–R, smaller θM–Land smaller θM–L/θM–R ratio. (2) The RF of RPA and the RF of LPA could be predicted with and without known MPA flow parameters in rTOF patients. (2a) With MPA flow parameters, both the RF of RPA and the RF of LPA can be predicted by MPA flow parameters and morphometrics. (2b) Without known MPA flow parameters, both can still be predicted by morphometrics alone with a less accuracy.
The underlying mechanism of complicative dynamic interactions of pulmonary arteries, ventricular function and lung condition is still not underscored. The proposed one by McElhinney et al. [12] sugested that (1). PR after repair operation caused the dilatation and elongation of the pulmonary trunk, (2). The right ventricular outflow tract would be transferred superiorly and rotated to the left. This causes the kinking of LPA at its origin from MPA, which may be coordinated by tethering of LPA by the ligamentum arteriosum. As a result, θM–L is smaller in rTOF patients than in the normal population. Besides, θM–R becomes more obtuse, causing the θM–L/θM–R ratio smaller in rTOF patients. Kato et al. [9] also indicated that due to the limited anteroposterior mediastinal space, the enlargement of RV will cause the heart bulging into the left hemithorax and compressing the left lung, which implies the change in branch pulmonary artery angle with the kinking of LPA. The CT-based 3D morphometrics study by Luo et al. [13] showed that θM–R was larger and θM–L was smaller in rTOF patients as compared with the normal control group.
We found that none of CSA of all three pulmonary arteries correlates with the RF of LPA, while the bifurcation angles are the key factors. Chern et al. have used an in-vitro numerical pulmonary artery model based on computational fluid dynamics to simulate flow variations in pulmonary arteries after repair of TOF [10]. They showed that regurgitation of LPA occurs earlier than RPA and MPA when θM–R was set significantly more obtuse than θM–L, which implied that the regurgitation of LPA may be more prolonged and prominent than RPA in that situation. Previous studies have established that theoretically there are optimal values for vascular angles and diameters that make the arterial bifurcation more efficient physiologically [15, 16]. The optimal diameters and branching angles of pulmonary arteries are those that produce the minimal value of cost to drive the blood flow [17]. Zhang et al. [18] found that acute LPA angle is related to adverse hemodynamic performance, such as vortices formation, relative high wall shear stress, decreased flow distribution to the left lung and increased energy loss. Kinking or stenosis of the branch pulmonary artery would increase right ventricular afterload, contribute to vortices formation in the center of the vessel lumen, and cause the acceleration of pulmonary regurgitation. Echocardiography study by Zhang et al. [14] also showed that the global longitudinal strain of RV was lower for acute LPA angulation than for round and blunt LPA angulation in rTOF patients. Our findings strongly support the concept that the sharper bifurcation angle of LPA is the key factor of increased RF in LPA of rTOF patients.
In contrary to LPA, the RF of RPA was correlated with CSA of RPA, consistent with our previous study [7]. Harris et al. [19] observed a significantly increased RF in the larger branching pulmonary artery as compared with the smaller one, a finding that aligns with our results concerning the RF of RPA. Kato et al. [9] found that the left lung area ratio correlated inversely with the RF of LPA and α angle. As the heart gradually enlarged, it would rotate into the left chest, compressing the left lung. We postulated that the enlarged heart would influence the RF of LPA, RPA, and even MPA, according to the results of this study (Table 4).
The best time for performing total repair of TOF is still controversial in pediatric cardiovascular surgery. The American Association for Thoracic Surgery2022 Expert Consensus Document by Miller et al. [20] concluded that the best age for total repair of TOF lies between 3 and 6 months for asymptomatic infants. However, Tamesberger et al. [21] found that neonatal total repair is associated with more frequent use of transannular patches and reinterventions, which may cause the subsequent variable degree of PR. Borowski et al. [22] found that rTOF patients undergoing transannular patch aged > 5 years had considerably longer redo-free intervals than their younger counterparts, which implied that the younger patients may suffer from more severe degree of PR. These studies may support our findings that total repair performed at a later age is correlated with a reduced degree of RF in the MPA, LPA, and RPA, as revealed through univariate analysis in this study.
In model 1 (incorporating MPA flow parameters) of this study, the RF of both RPA and LPA can be predicted by MPA flow parameters and morphology. In model 2 (excluding MPA flow parameters), the pulmonary bifurcation angle is the key factor for the RF of LPA, whereas CSA of pulmonary arteries and left lung area ratio serve as key factors for the RF of RPA. Model 1 offers a stronger prediction due to the influence of MPA flow and pulmonary artery morphometrics; however, model 2 provides simply morphometrical information for predicting PR in instances where flow parameters are unavailable, such as when the computed tomography angiography is employed in the rTOF patients.
Based on the findings from current and previous studies [9, 19, 23], we postulated the consequent pathophysiology of PR in rTOF patients as follows: After total repair of TOF, relief of RV outflow tract obstruction may result in PR (mainly after transannular repair of TOF). Chronic volume overload from PR leads to RV dilatation and MPA elongation. RV dilatation combined with subsequent left ventricular dilatation prompts the enlarged heart to rotate into the left chest, then compressing the left lung. This compression increases left pulmonary vascular resistance, further augmenting diastolic flow reversal in the LPA [19], and assumedly in the MPA and RPA as well. Besides, MPA elongation causes LPA kinking, consequently increasing the RV afterload and exacerbating PR. As a result, it is crucial to monitor the morphometric configuration of pulmonary arteries and the RV during long-term follow-up after TOF repair in patients with significant pulmonary regurgitation, even when prior assessments revealed no apparent abnormalities.
During surgical intervention, the influence and importance of the branching pulmonary artery angles should be considered. Luo et al. [13] showed that a larger preoperative pulmonary artery bifurcation angle is a morphometric predictor for early reoperation in rTOF patients. To correct the kinking of the LPA with or without stenosis, it is sometimes necessary to remove redundancy at the kink point and reimplant the LPA at a more favorable vascular angle. In most of the time, it is also essential to shorten the dilated and elongated pulmonary trunk. If the ligamentum arteriosum remains intact, it should be separated, as it would restrict the mobility of the LPA and act as a pivot for the twisting of the dilated MPA [12]. Some surgeons have reported that adopting the pulmonary artery angioplasty technique, which utilizes the anterior wall flap of the MPA [24, 25], can effectively deal with acute angles and/or stenosis of the LPA during total repair of TOF; our findings agree with their suggestions.
The current study has some limitations. Firstly, the case number of this study is medium, which is insufficient to be age-based subgroup analysis. Increasing case number of different ages may reveal different morphometric predictors in different stage of age. Second, although we found an association between the morphometrics and the RF of LPA and RPA, the causal relationship is not clear. Conducting a longitudinal study on the same cases may provide a better interpretation. Third, we used a single axial plane to represent the lung and cardiac ventricular volume instead of 3D volume measurement of the whole lung and heart. However, we aimed to employ accepted method and keep our method simple and referrable. Forth, we used conventional 2D phase-contrast flow measurement. Future study using 4D flow may disclose novel parameters that carry better clinical impact. Fifth, the mechanisms of regurgitant flow may be influenced by other unchecked factors, such as peripheral pulmonary arterial resistance, lung parenchymal pathology, restrictive physiology of RV, etc., which were not investigated in this study.
Conclusions
In rTOF patients, the RF of LPA is more severe than that of RPA, which may be related to the vascular morphometrics. Our study indicates that a reduced ratio of θM–L/θM–R highly associated with increase of RF of the LPA, which may offer potential insights for surgical strategies and follow-up management in patients with TOF.
Data and Code availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3D:
-
3-dimensional
- BFV:
-
Backward flow volume
- CE-MRA:
-
Contrast-enhanced magnetic resonance angiography
- CMR:
-
Cardiovascular magnetic resonance
- CSA:
-
Cross-sectional area
- FFV:
-
Forward flow volume
- LPA:
-
Left pulmonary artery
- MPA:
-
Main pulmonary artery
- NFV:
-
Net flow volume
- PC-MRI:
-
Phase-contrast magnetic resonance imaging
- PR:
-
Pulmonary regurgitation
- RF:
-
Regurgitant fraction
- RPA:
-
Right pulmonary artery
- rTOF:
-
Repaired tetralogy of fallot
- RV:
-
Right ventricle
- α angle:
-
The angle between the thoracic anterior–posterior line and the interventricular septum
- θL –AP :
-
The angle between the thoracic anterior–posterior line and left pulmonary artery
- θM –AP :
-
The angle between the thoracic anterior–posterior line and main pulmonary artery
- θM –L :
-
The angle between main pulmonary artery and left pulmonary artery
- θM –R :
-
The angle between main pulmonary artery and right pulmonary artery
- θR –AP :
-
The angle between the thoracic anterior–posterior line and right pulmonary artery
References
Murphy JG, Gersh BJ, Mair DD et al (1993) Long-term outcome in patients undergoing surgical repair of tetralogy of fallot. N Engl J Med 329(9):593–599
Gatzoulis MA, Balaji S, Webber SA et al (2000) Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of fallot: a multicentre study. Lancet 356(9234):975–981
Norton KI, Tong C, Glass RBJ, Nielsen JC (2006) Cardiac MR imaging assessment following tetralogy of fallot repair. Radiographics 26(1):197–211
Wald RM, Redington AN, Pereira A et al (2009) Refining the assessment of pulmonary regurgitation in adults after tetralogy of fallot repair: should we be measuring regurgitant fraction or regurgitant volume? Eur Heart J 30(3):356–361
Sridharan S, Derrick G, Deanfield J, Taylor AM (2006) Assessment of differential branch pulmonary blood flow: a comparative study of phase contrast magnetic resonance imaging and radionuclide lung perfusion imaging. Heart 92(7):963–968
Kang IS, Redington AN, Benson LN et al (2003) Differential regurgitation in branch pulmonary arteries after repair of tetralogy of fallot: a phase-contrast cine magnetic resonance study. Circulation 107(23):2938–2943
Wu M-T, Huang Y-L, Hsieh K-S et al (2007) Influence of pulmonary regurgitation inequality on differential perfusion of the lungs in tetralogy of fallot after repair. J Am Coll Cardiol 49(18):1880–1886
Harris MA, Weinberg PM, Whitehead KK, Fogel MA (2005) Usefulness of branch pulmonary artery regurgitant fraction to estimate the relative right and left pulmonary vascular resistances in congenital heart disease. Am J Cardiol 95(12):1514–1517
Kato A, Drolet C, Yoo S-J, Redington AN, Grosse-Wortmann L (2016) Vicious circle between progressive right ventricular dilatation and pulmonary regurgitation in patients after tetralogy of fallot repair? Right heart enlargement promotes flow reversal in the left pulmonary artery. J Cardiovasc Magn Reson 18(1):34. https://doi.org/10.1186/s12968-016-0254-1
Chern MJ, Wu MT, Her SW (2012) Numerical study for blood flow in pulmonary arteries after repair of tetralogy of fallot. Comput Math Methods Med. https://doi.org/10.1155/2012/198108
Rahkonen O, Chaturvedi RR, Benson L, Honjo O, Caldarone CA, Lee KJ (2015) Pulmonary artery stenosis in hybrid single-ventricle palliation: high incidence of left pulmonary artery intervention. J Thorac Cardiovasc Surg 149(4):1102-1110.e1102
McElhinney DB, Parry AJ, Reddy VM, Hanley FL, Stanger P (1998) Left pulmonary artery kinking caused by outflow tract dilatation after transannular patch repair of tetralogy of fallot. Ann Thorac Surg 65(4):1120–1126
Luo Q, He X, Song Z et al (2021) Preoperative morphological prediction of early reoperation risk after primary repair in tetralogy of fallot: a contemporary analysis of 83 cases. Pediatr Cardiol 42(7):1512–1525
Zhang S, He X, Liu L et al (2021) Assessing right ventricular systolic function using ultrasonic speckle-tracking imaging in repaired tetralogy of fallot with different pulmonary artery branch angles. Echocardiography 38(1):89–96
Zamir M, Bigelow DC (1984) Cost of departure from optimality in arterial branching. J Theor Biol 109(3):401–409
Woldenberg MJ, Horsfield K (1986) Relation of branching angles to optimality for four cost principles. J Theor Biol 122(2):187–204
Fanucci E, Orlacchio A, Pocek M, Magrini A, Salomoni E (1990) Optimal branching of human arterial bifurcations. Invest Radiol 25(1):62–66
Zhang W, Liu J, Yan Q, Liu J, Hong H, Mao L (2016) Computational haemodynamic analysis of left pulmonary artery angulation effects on pulmonary blood flow. Interact Cardiovasc Thorac Surg 23(4):519–525
Harris MA, Whitehead KK, Gillespie MJ et al (2011) Differential branch pulmonary artery regurgitant fraction is a function of differential pulmonary arterial anatomy and pulmonary vascular resistance. JACC Cardiovasc Imaging 4(5):506–513
Miller JR, Stephens EH, Goldstone AB et al (2023) The American association for thoracic surgery (AATS) 2022 expert consensus document: management of infants and neonates with tetralogy of fallot. J Thorac Cardiovasc Surg 165(1):221–250
Tamesberger MI, Lechner E, Mair R, Hofer A, Sames-Dolzer E, Tulzer G (2008) Early primary repair of tetralogy of fallot in neonates and infants less than four months of age. Ann Thorac Surg 86(6):1928–1935
Borowski A, Ghodsizad A, Litmathe J, Lawrenz W, Schmidt KG, Gams E (2004) Severe pulmonary regurgitation late after total repair of tetralogy of fallot: surgical considerations. Pediatr Cardiol 25(5):466–471
Lee C (2012) Surgical management of chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction. Korean Circ J 42(1):1–7
Kim H, Sung SC, Chang YH, Lee HD, Park JA (2014) Early and midterm outcomes of left pulmonary artery angioplasty using an anterior wall flap of the main pulmonary artery in tetralogy of fallot repair. J Thorac Cardiovasc Surg 148(6):2597–2601
Jang WS, Kim W-H, Cho S (2017) Effects of angle correction angioplasty for pulmonary artery stenosis with tetralogy of fallot. Ann Thorac Surg 103(3):862–868
Acknowledgements
The authors appreciated Siou-Fong Pan and research assistant Chiung-Chih Hu for data management.
Funding
Open Access funding enabled and organized by National Yang Ming Chiao Tung University. This research was supported by Veterans General Hospitals and University System of Taiwan Joint Research Program (VGHUST108-G3-3–2, VGHUST109-V3-3–3, KSVGH112-022).
Author information
Authors and Affiliations
Contributions
MTW conceptualized and designed the study; YCC, YLC, MHC, and HWC contributed to data acquisition; HCT and MTW performed data analysis and interpreted the data; HCT drafted the manuscript; MTW and KPW revised the manuscript; MTW and JYP performed scientific and clinical supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This research was approved by Kaohsiung Veterans General Hospital Institutional Review Board (VGHKS14-CT1-16).
Consent to participate
Written informed consent was obtained from the patients or patients' parents, depending on the patients’ age.
Consent to publish
The parents signed informed consent regarding publishing the data and photographs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teng, HC., Chen, YC., Chen, YL. et al. Morphometrics predicts the differential regurgitant fraction in bilateral pulmonary arteries of patients with repaired tetralogy of fallot. Int J Cardiovasc Imaging 40, 655–664 (2024). https://doi.org/10.1007/s10554-023-03035-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-03035-1